18 F-labeled affimer compound and its preparation method and application
A compound and labeling technology, applied in the field of 18F-labeled affinity compound and its preparation, can solve the problems of low tumor sensitivity, complex preparation method, large liver toxicity and side effects, etc., and achieve high radiochemical purity, short residence time, toxicity and so on. Effects with few side effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
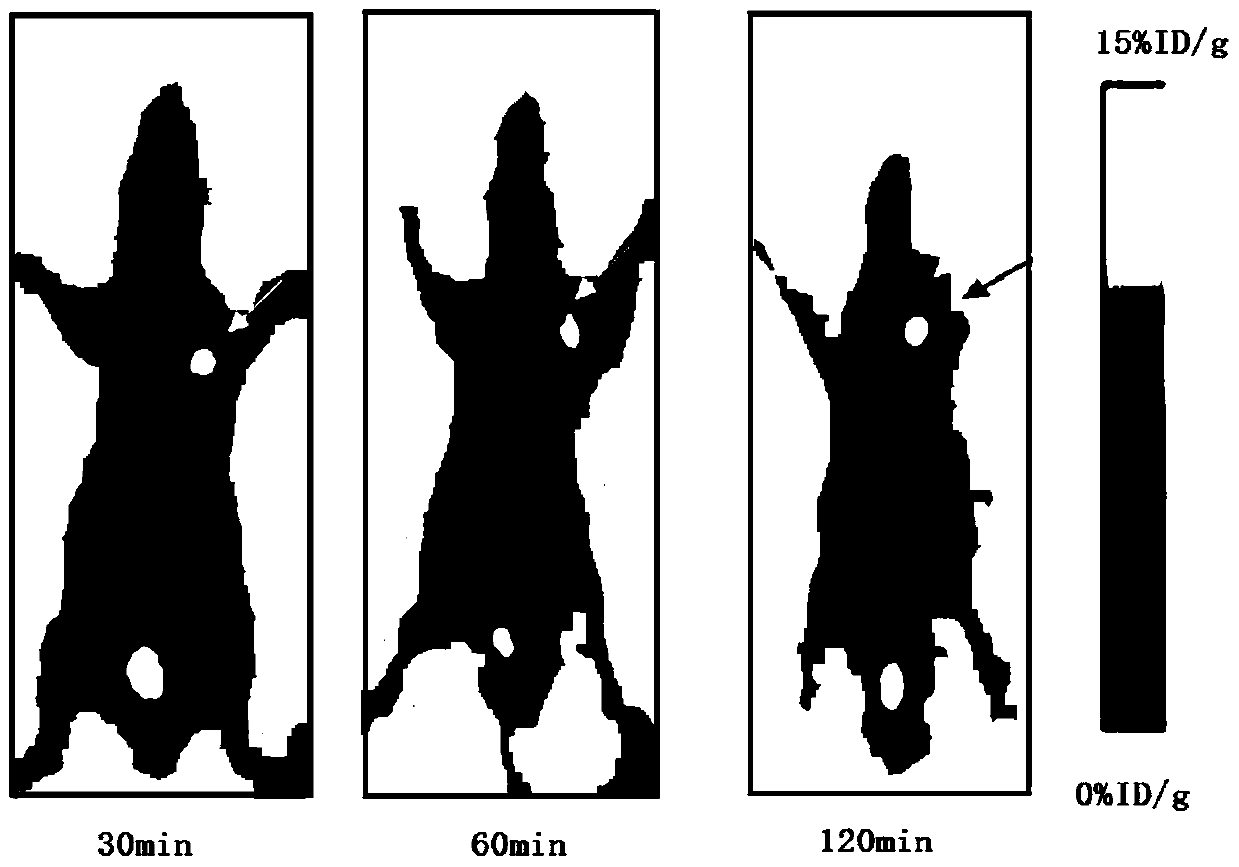
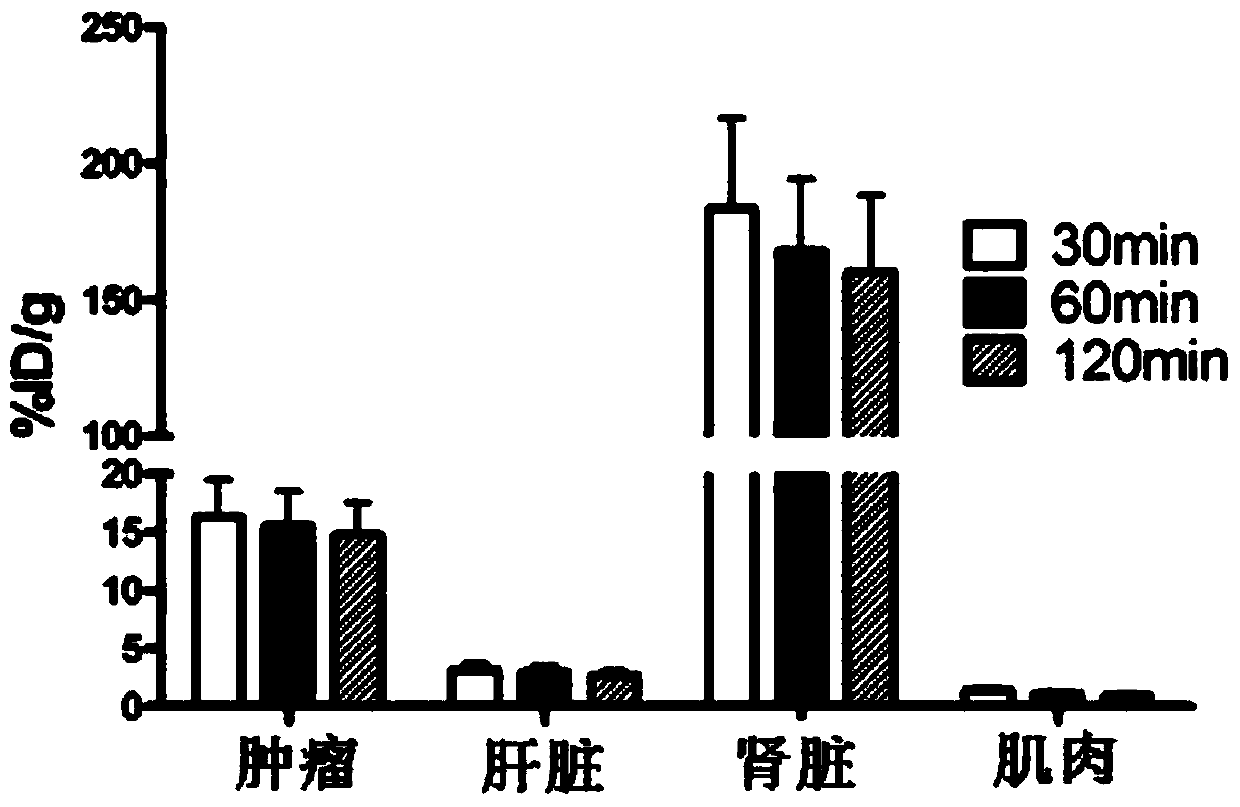
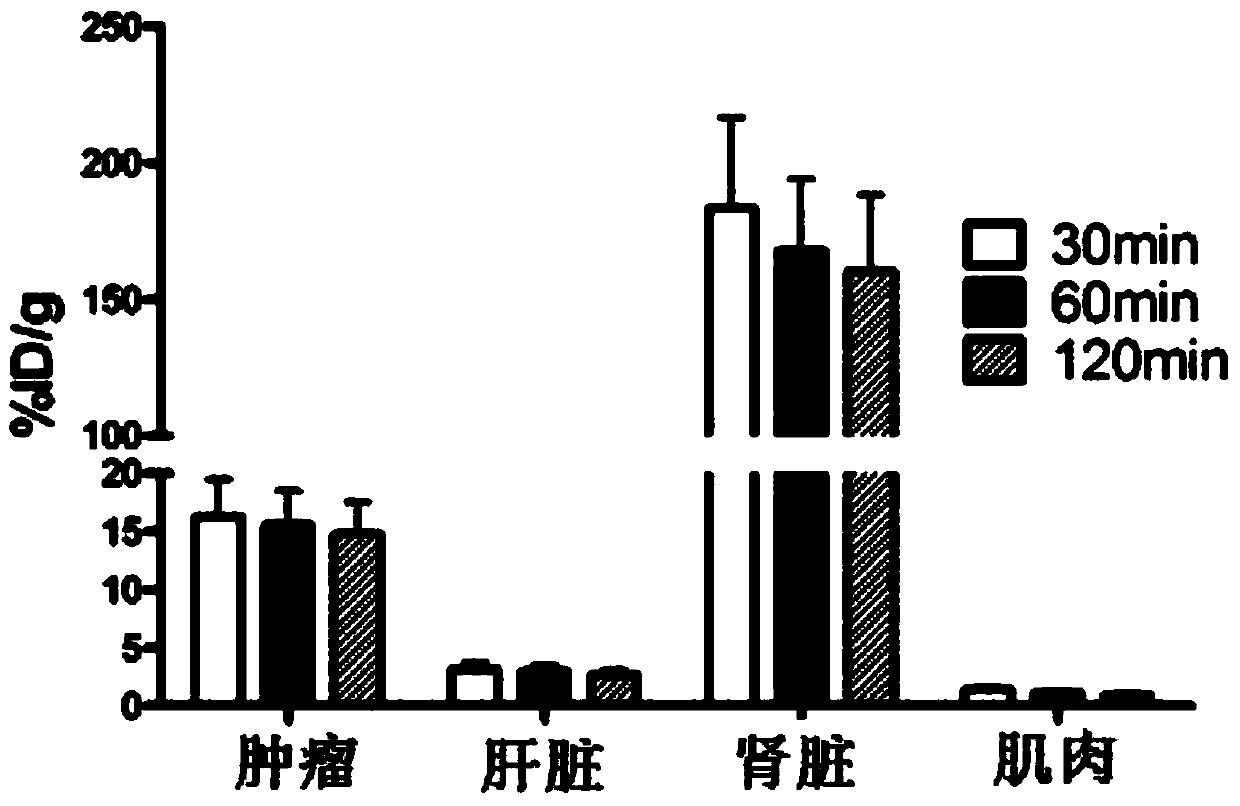
Image
Examples
Embodiment 1
[0059] This embodiment is used in the kit for the preparation of the compound shown in formula (I), and its reagents include: 50nmol of the compound shown in formula (II), 12nmol of aluminum chloride, 0.5mol / L, acetic acid- Sodium acetate buffer;
[0060] The preparation method of the medicine box comprises the following steps:
[0061] (1) Dissolving the selected mole of the compound shown in formula (II) in 0.5 mol / L, pH 4.0 acetic acid-sodium acetate buffer solution to obtain solution A for subsequent use;
[0062] (2) dissolving selected moles of aluminum chloride in acetic acid-sodium acetate buffer solution of 0.5mol / L and pH of 4.0 to obtain solution B for subsequent use;
[0063] (3) Mix the solution A and the solution B uniformly to obtain the solution C for subsequent use;
[0064] (4) The solution C is subjected to sterile filtration, divided into controlled antibiotic bottles, freeze-dried for 24 hours, and sealed with a stopper to obtain the product.
[0065] T...
Embodiment 2
[0070] This embodiment is used in the kit for preparing the compound shown in formula (I), and its reagent comprises: 1nmol compound shown in formula (II), 1nmol aluminum trichloride, 0.4mol / L, the acetic acid- Sodium acetate buffer;
[0071] The preparation method of the medicine box comprises the following steps:
[0072] (1) Dissolving the selected mole of the compound shown in formula (II) in 0.4mol / L, pH 5.0 acetic acid-sodium acetate buffer solution to obtain solution A for subsequent use;
[0073] (2) dissolving selected moles of aluminum chloride in acetic acid-sodium acetate buffer solution of 0.4mol / L and pH of 5.0 to obtain solution B for subsequent use;
[0074] (3) Mix the solution A and the solution B uniformly to obtain the solution C for subsequent use;
[0075] (4) The solution C is subjected to sterile filtration, divided into controlled antibiotic bottles, freeze-dried for 24 hours, and sealed with a stopper to obtain the product.
[0076] The preparation...
Embodiment 3
[0081] This embodiment is used in the kit for preparing the compound shown in formula (I), and its reagent comprises: 5nmol compound shown in formula (II), 1nmol aluminum trichloride, 0.6mol / L, the acetic acid- Sodium acetate buffer;
[0082] The preparation method of the medicine box comprises the following steps:
[0083] (1) Dissolving the selected mole of the compound shown in formula (II) in 0.6mol / L, pH 3.0 acetic acid-sodium acetate buffer solution to obtain solution A for subsequent use;
[0084] (2) dissolving selected moles of aluminum chloride in acetic acid-sodium acetate buffer solution of 0.6mol / L and pH of 3.0 to obtain solution B for subsequent use;
[0085] (3) Mix the solution A and the solution B uniformly to obtain the solution C for subsequent use;
[0086] (4) The solution C is subjected to sterile filtration, divided into controlled antibiotic bottles, freeze-dried for 24 hours, and sealed with a stopper to obtain the product.
[0087] The preparation...
PUM
| Property | Measurement | Unit |
|---|---|---|
| quality score | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com