Preparation method for nano-alloy catalyst
A nano-alloy and catalyst technology, which is applied in the application field of nano-alloy catalysts in fuel cells, can solve the problems of cumbersome preparation process, methanol loss performance, and poor environmental protection, and achieve high activity, good stability, and safe and environmentally friendly reagents.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
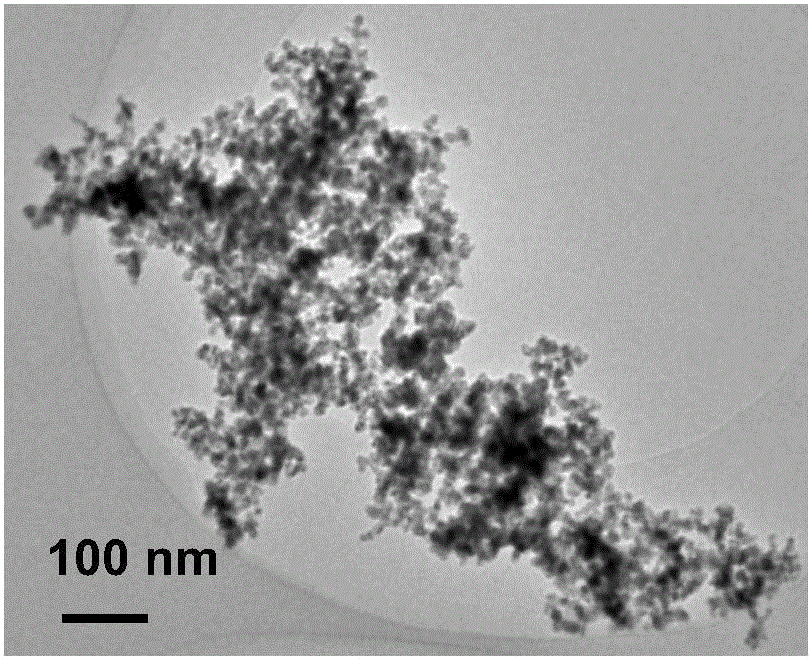
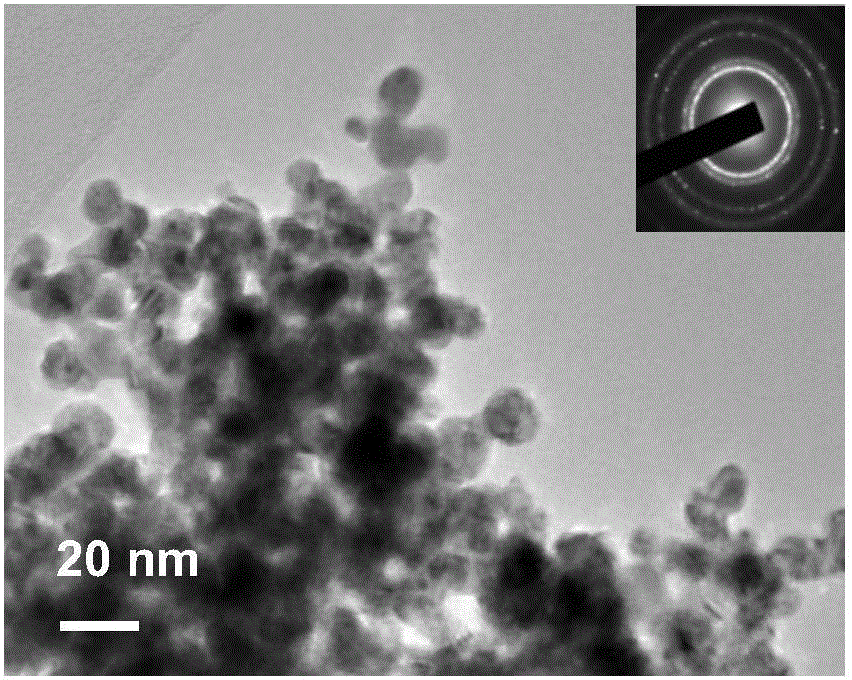
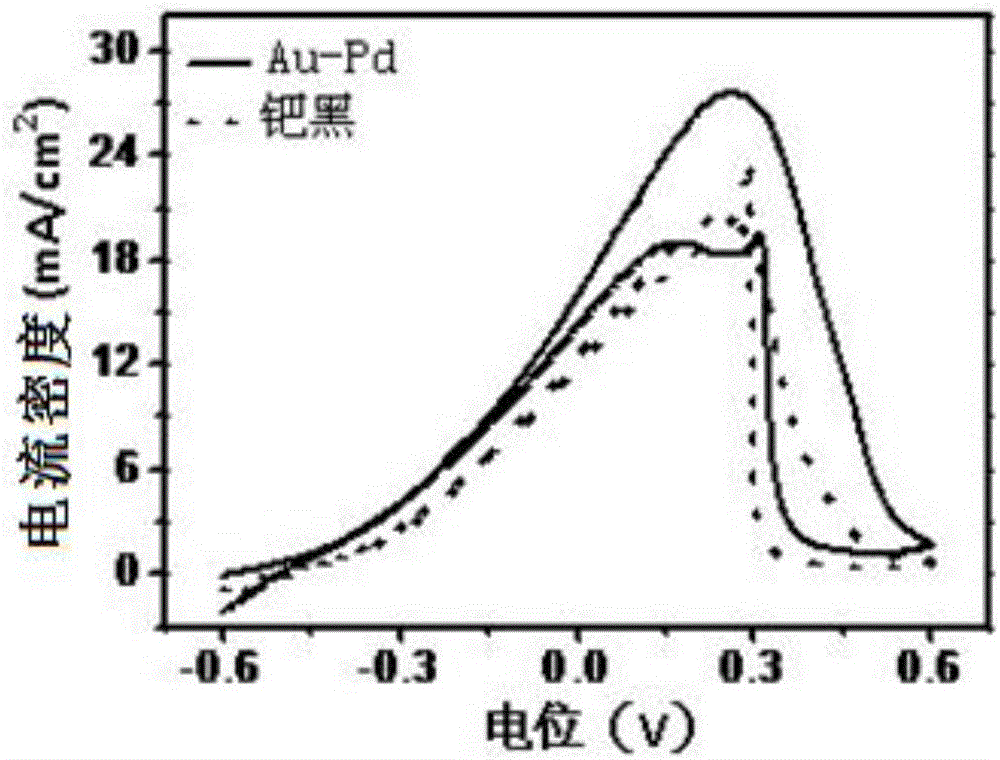
Image
Examples
Embodiment 1
[0032] Step 1, preparation of AuPd nanocatalyst
[0033] Reagent: KOH: 1mol / L; HAuCl 4 : 24.3mmol / L; PdCl 2 : 100mmol / L; hydrazine hydrate: 16.7mmol / L.
[0034] Dissolve 0.0428g aminoorotic acid in 1mL KOH solution for later use. Take 0.823mL HAuCl 4 solution and 0.5mL PdCl 2 The solution was placed in a 20mL beaker, and amino orotic acid-base solution and 7.677mL of secondary water were added, and magnetically stirred at a constant temperature of 15°C for half an hour. Then, 0.1 mL of hydrazine hydrate was added dropwise into the beaker, and magnetic stirring was continued at a constant temperature of 20° C. for 1 hour to obtain a suspension. Add secondary water to the obtained suspension, centrifuge and wash the precipitate 5 times until the supernatant of the washing liquid is clear, transparent and colorless, then add ethanol to the precipitate, centrifuge, wash 3 times, collect the black solid, 60 °C and dried for 12 hours to obtain the AuPd nanocatalyst.
[0035] ...
Embodiment 2
[0048] Step 1, preparation of AuPt nano-catalyst
[0049] Reagent: HAu(NO 3 ) 4 : 24.3mmol / L; H 2 PtCl 6 : 100mmol / L.
[0050] First configure 0.5% undecyltriazine sodium carbonate solution and 0.1mol / L ascorbic acid solution for later use. Take 5mL undecyltriazine solution and place it in a 20mL beaker at 30°C and stir, then add 0.823mL HAu(NO 3 ) 4 solution and 0.518mL H 2 PtCl 6 The solution was stirred for 10 min, then 1 mL of ascorbic acid solution and 2.659 mL of secondary water were added, and magnetically stirred at a constant temperature of 30° C. for 1 hour to obtain a suspension. Add secondary water to the suspension obtained from the reaction, centrifuge and wash the precipitate 5 times until the supernatant of the washing liquid is clear, transparent and colorless, then add ethanol to the precipitate, centrifuge and wash 3 times, and collect the black solid. Dry at 40°C for 48 hours to obtain the AuPt nanocatalyst.
[0051] 2 mg of the obtained AuPt nano...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com