Cup-type time-resolved fluorescent immunoassay kit for high-sensitivity C-reactive protein based on microspheres, and preparation method and application thereof
A time-resolved fluorescence and protein-reactive technology, which is applied in the field of clinical medical diagnosis, can solve the problems of difficult routine implementation in clinical laboratories, unsatisfactory sensitivity and specificity, and high technical requirements for chemiluminescence, so as to achieve easy automatic operation and shorten detection time , The effect of improving the detection sensitivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0044] (1) Preparation of detection cuvette:
[0045] A. Detection cuvette coating: C-reactive protein antibody 6404 (Medix Company) was diluted to 10 μg / ml with 0.2mol / L phosphate buffer (pH7.8), 100 μL / well, coated at 37°C for two hours, and washed. .
[0046] B. Detection cuvette blocking: Add 200 μL / well of blocking buffer to the cuvette, block at 37°C for two hours, discard the blocking solution in the coated cuvette, and pat dry.
[0047] C. Drying of the test cuvette: place the sealed cuvette above in a 37° C. drying oven with a humidity lower than 30% for 4 hours, and store in a sealed and dry place.
[0048] (2) Preparation of fluorescently labeled antibodies:
[0049] A. Fluorescent particle activation:
[0050] Take 1mg of carboxyl time-resolved fluorescent microspheres (300nm, 0.1ml, 10mg / mL, Bangslab Company), add 60μL of 500mmol / L MES (2-(N-morpholine)ethanesulfonic acid) buffer, pH 6.0, add Add purified water to 0.2mg 1-(3-dimethylaminopropyl)-3-ethylcarbodi...
Embodiment 2
[0060] (1) Drawing of standard curve:
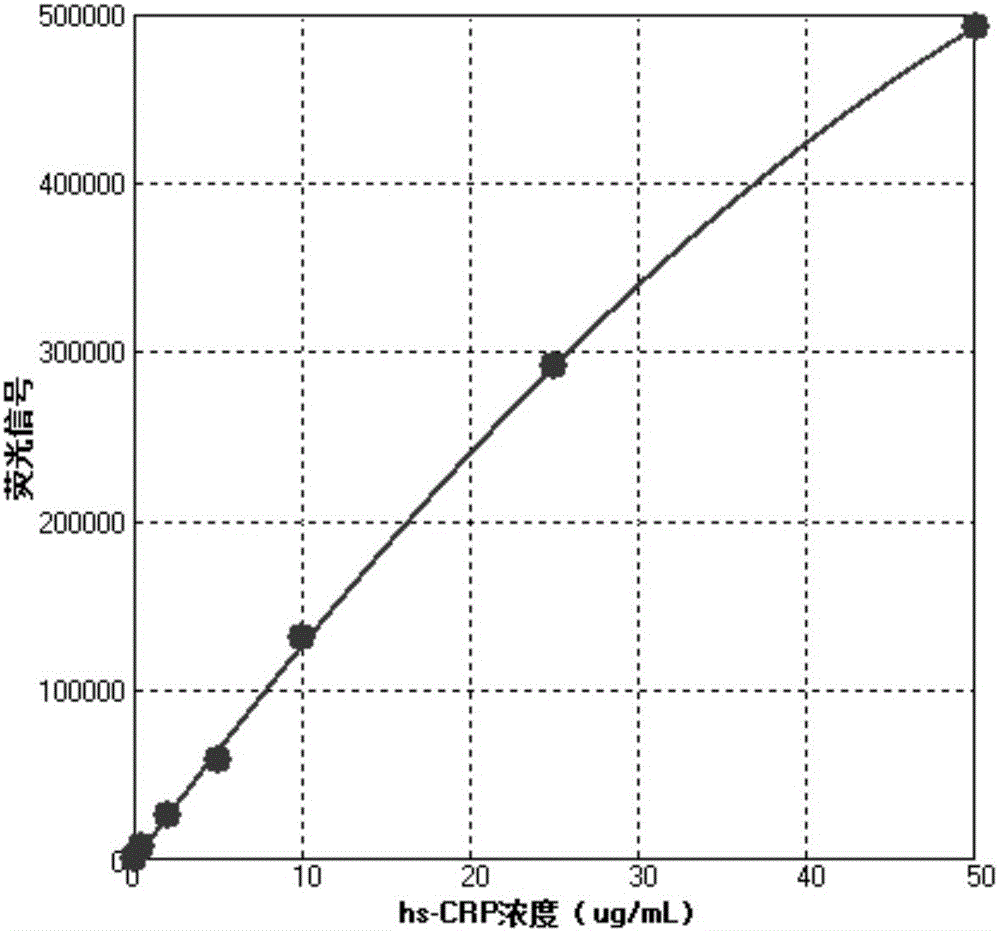
[0061] The reagents prepared in Example 1 were combined into a high-sensitivity C-reactive protein time-resolved fluorescence quantitative kit, and the calibrator was measured, and each concentration was repeated 10 times.
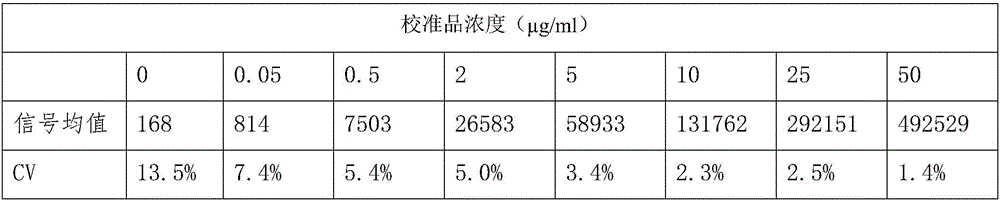
[0062] Add 3 μL of calibrator and 100 μL of fluorescently labeled antibody to each detection, incubate at 37°C for 20 min, then wash the detection cup, and read on the Victor X4 fluorescence reader from PerkinElmer (excitation wavelength 340-380nm, detection wavelength 600-630nm) The specific data are shown in Table 1.
[0063] Table 1
[0064]
[0065] According to the data in Table 1, the concentration of the calibrator is taken as the abscissa, and the mean fluorescence signal is taken as the ordinate to draw a standard curve. standard curve as figure 2 shown. The standard curve has good linearity, and the concentration of high-sensitive C-reactive protein contained in the sample can be quantitatively anal...
Embodiment 3
[0073] (1) Preparation of detection cuvette:
[0074] A. Detection cuvette coating: C-reactive protein antibody 6404 (Medix Company) was diluted to 10 μg / ml with 0.2mol / L phosphate buffer (pH7.8), 100 μL / well, coated at 37°C for two hours, and washed. .
[0075] B. Detection cuvette blocking: Add 200 μL / well of blocking buffer to the cuvette, block at 37°C for two hours, discard the blocking solution in the coated cuvette, and pat dry.
[0076] C. Drying of the test cuvette: place the sealed cuvette above in a 37° C. drying oven with a humidity lower than 30% for 4 hours, and store in a sealed and dry place.
[0077] (2) Preparation of fluorescently labeled antibodies:
[0078] A. Fluorescent particle activation:
[0079] Take 1mg of carboxyl time-resolved fluorescent microspheres (300nm, 0.1ml, 10mg / mL, Bangslab company), add 60ul 500mM MES (2-(N-morpholine)ethanesulfonic acid) buffer, pH 6.0, add 0.2mg 1 -(3-Dimethylaminopropyl)-3-ethylcarbodiimide hydrochloride (EDC), a...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com