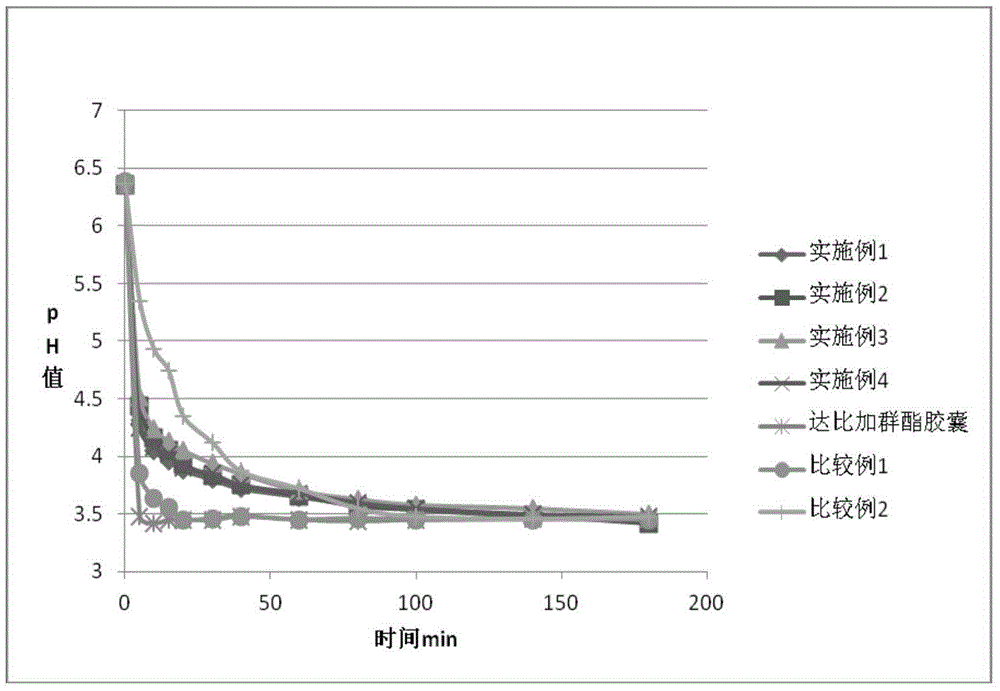
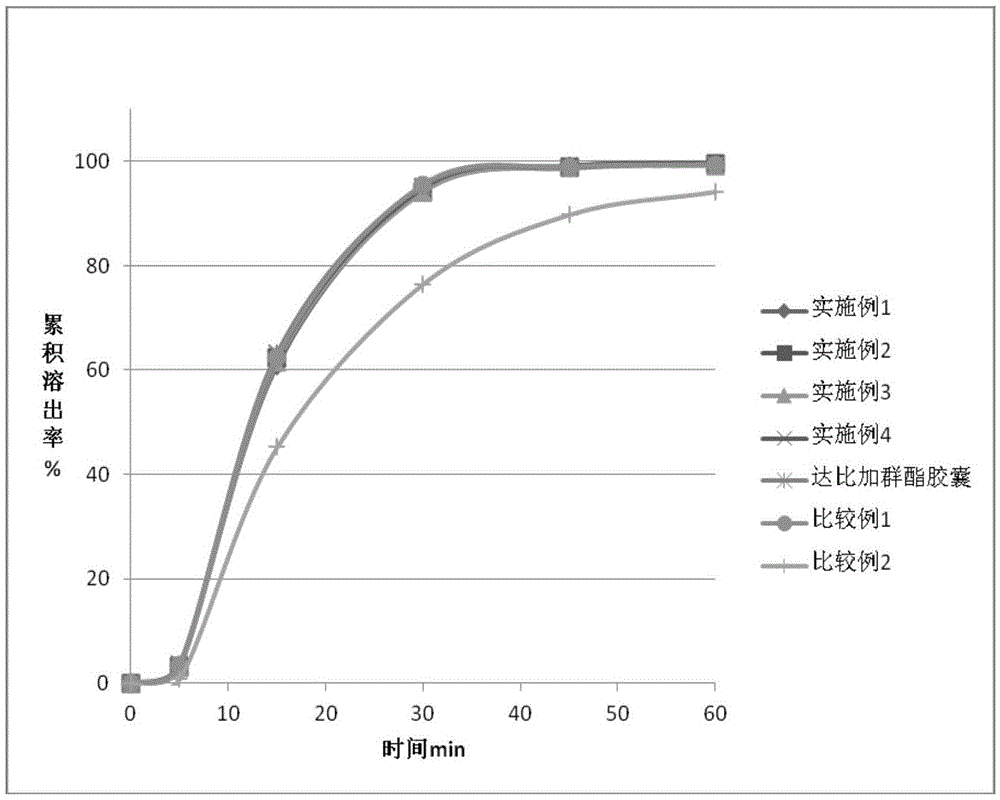
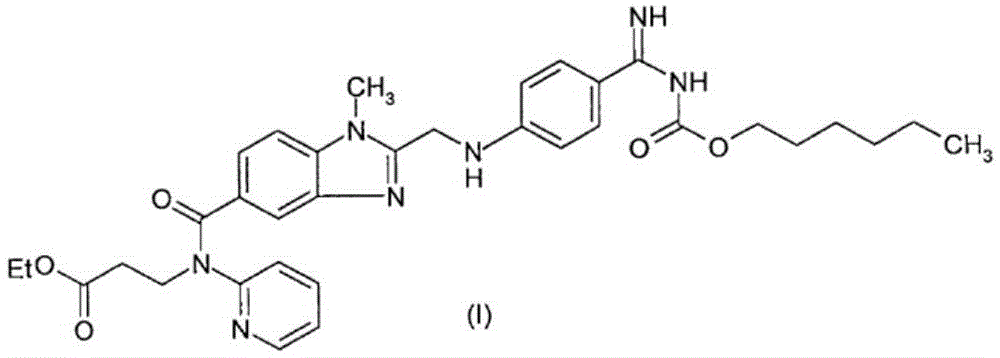
Dabigatran tablet, and preparation method and application thereof
A technology for dabigatran etexilate and gatran etexilate tablets, which is applied in the field of dabigatran etexilate tablets and its preparation, can solve the problems of high requirements, damage to the gastrointestinal tract, cumbersome operations, etc., and reduce production costs , reduce irritation and damage, and achieve the effect of industrialization
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0071] The present invention also provides a kind of preparation method of dabigatran etexilate tablet, comprising:
[0072] A) uniformly mixing the organic acid and the sustained-release material and dry granulating to obtain organic acid granules;
[0073] B) mixing dabigatran etexilate and pharmaceutically acceptable salts thereof and auxiliary materials and dry granulating to obtain dabigatran etexilate granules;
[0074] C) the organic acid granules and the dabigatran etexilate granules are compressed by a double-layer tablet press to obtain a double-layer tablet; or by a three-layer tablet press, the middle layer is the dabigatran etexilate layer, and the other two The first layer is an organic acid layer to obtain a three-layer tablet; or the tablet is compressed by a three-layer tablet machine, the middle layer is an auxiliary material layer, and the remaining two layers are respectively an organic acid layer and a dabigatran etexilate layer to obtain a three-layer tab...
Embodiment 1
[0083] According to the ingredients listed in Table 1, the components of dabigatran etexilate and organic acid were made into granules by dry granulation, and then compressed into double-layer tablets, and then Opadry II film-coated premix was used The coating material is used for coating.
[0084] Table 1 embodiment 1 group distribution ratio
[0085]
Embodiment 2
[0087] According to the ingredients listed in Table 2, the components of dabigatran etexilate and organic acid were made into granules by dry granulation, and then compressed into double-layer tablets.
[0088] Table 2 Example 2 group distribution ratio
[0089]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com