A binder, its preparation method and a secondary battery containing the binder
A secondary battery and binder technology, applied in battery electrodes, circuits, organic chemistry, etc., can solve problems such as large swelling rebound, poor thermal stability, and potential safety hazards, and achieve high thermal stability, stable main chain structure, and improved Effect of Magnification Performance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
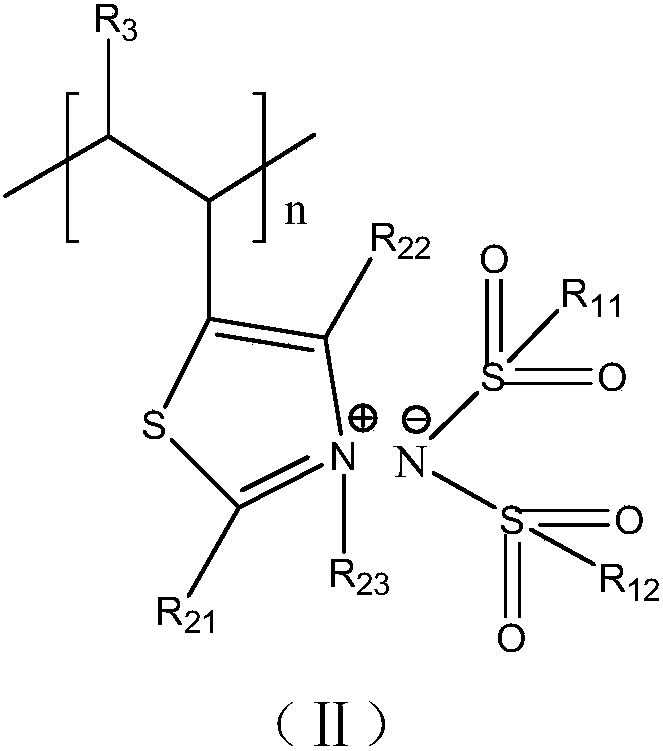
[0052] The present application also relates to the preparation method of the adhesive, the preparation method of the structural unit shown in formula II at least includes the following steps:
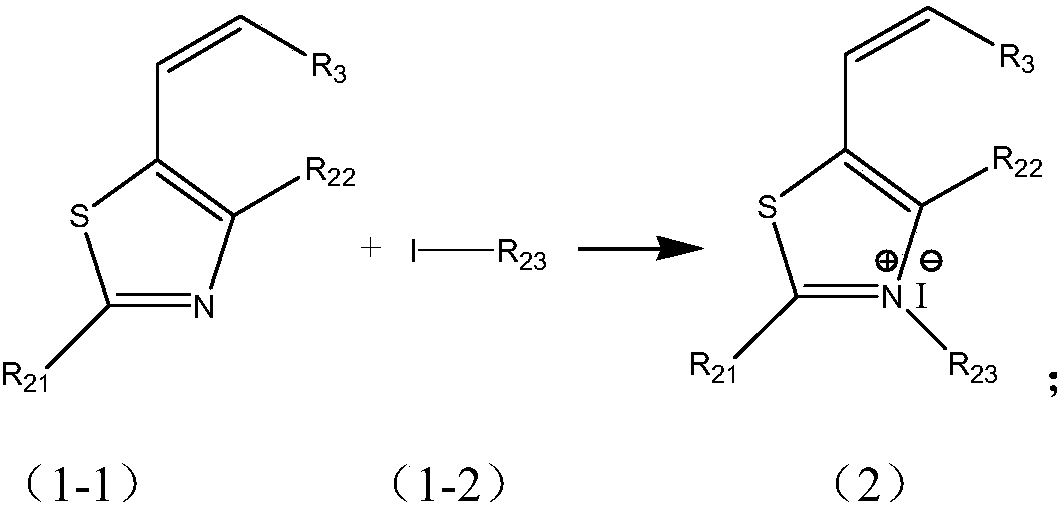
[0053] (1) Preparation of substituted 5-alkenylthiazole iodides;
[0054]
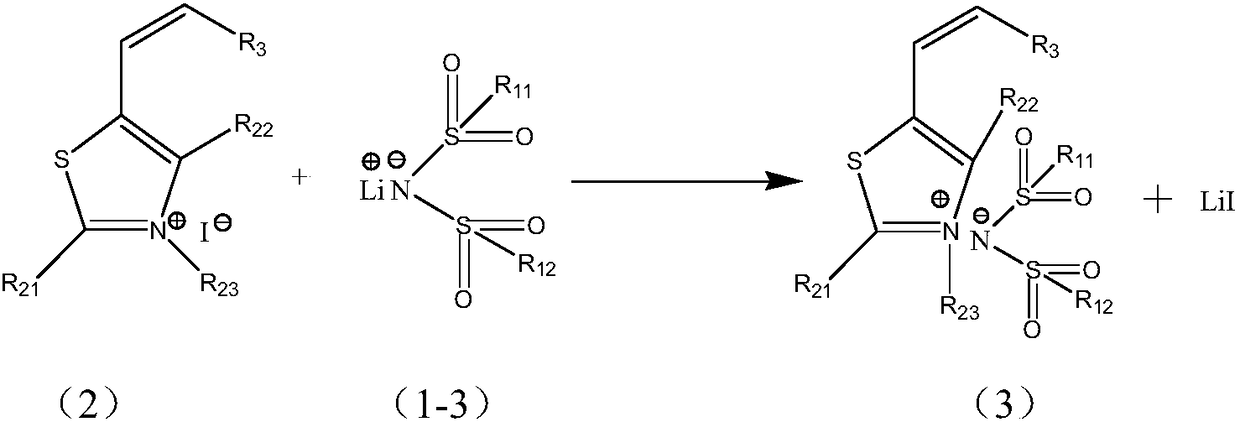
[0055] (2) Using a lithium salt with a hydrophilic group and thiazole iodide to carry out a displacement reaction to prepare a compound shown in formula 3;
[0056]
[0057] (3) The compound shown in formula 3 is polymerized to obtain the structural unit shown in formula II:
[0058]
[0059] Among them, R 11 , R 12 each independently selected from substituted C 1-6 Alkyl, the substituent is halogen;
[0060] R 21 , R 22 independently selected from hydrogen atom, C 1-12 Alkyl, C 1-12 Alkoxy or C 1-12 Alkanoyloxy;
[0061] R 23 from C 1-12 alkyl;
[0062] R 23 selected from halogen, C 1-6 alkyl.
[0063] Among them, the compounds represented by raw materials 1-1, 1-2, and 1-3 in this synthe...
Embodiment 1~8
[0072] The binders of Examples 1 to 8 are composed of structural units shown in Formula II, and the specific selection of substituents in Examples 1 to 8 is shown in Table 1:
[0073]
[0074] Table 1:
[0075] Example
R 11
R 12
R 21
R 22
R 23
R 3
Example 1
-CF 3
-CF 3
-H
-CH 3
-CH 3
-H
Example 2
-CH 2 CF 3
-CH 2 CF 3
-H
-CH 3
-CH 3
-H
Example 3
-CH 2 Cl
-CH 2 Cl
-H
-CH 3
-C 2 h 5
-H
Example 4
-C 2 f 5
-C 2 f 5
-CH 3
-C 2 h 5
-C 2 h 5
-Cl
Example 5
--CHFCH 2 f
-CH 2 f
-CH 3
-C 3 h 7
-C 3 h 7
-Cl
Example 6
-CH 2 CH 2 Cl
-CH 2 CH 2 Cl
-CH 3
-H
-C 4 h 9
-CH 3
Example 7
--CHFCH 2 f
-CH 2 CH 2 f
-C 2 h 5
-H
-C 5 h 11
-CH 3
Example 8
-C 3 f ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com