Plasma miRNA marker relevant to lung adenocarcinoma auxiliary diagnosis and application thereof
An auxiliary diagnosis and lung adenocarcinoma technology, applied in the fields of genetic engineering and oncology, can solve the problems of high price and inability to promote, the detection sensitivity and specificity need to be strengthened, and relying on the experience of the tester. Easy to obtain and stable effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
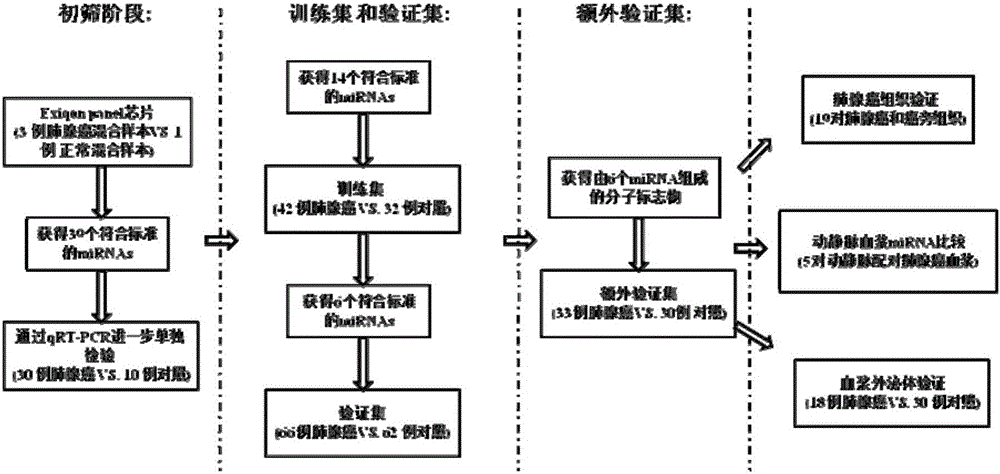
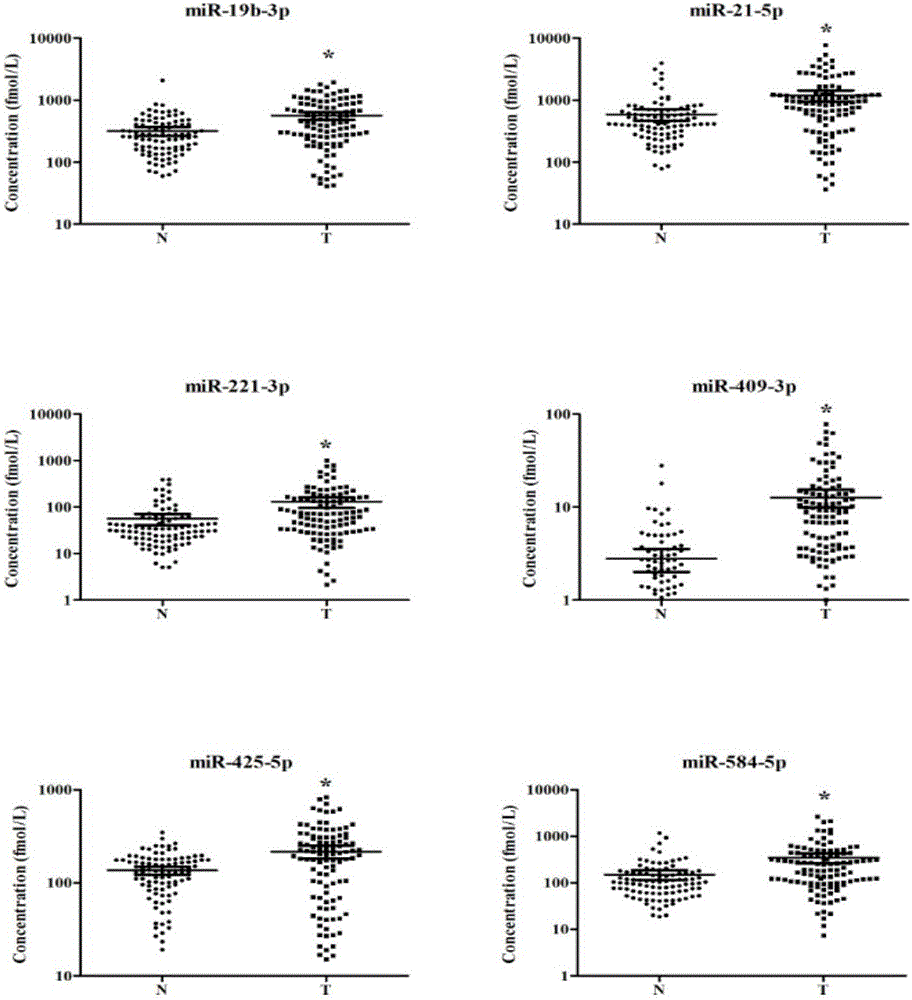
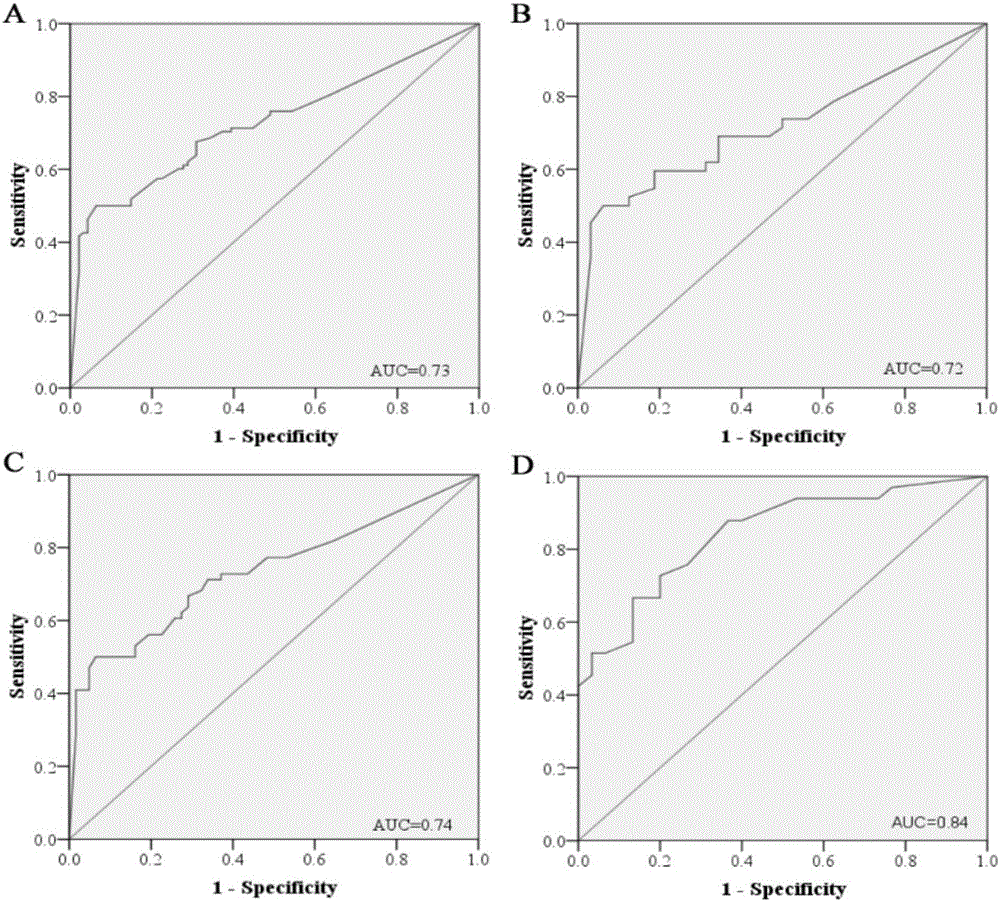
[0040] From 2012 to 2014, the inventor collected a large number of venous plasma samples from patients with lung adenocarcinoma and normal physical examination population from the First Affiliated Hospital of Nanjing Medical University, and selected 141 cases of lung adenocarcinoma and 124 cases of The normal control sample was used as the experimental sample for the initial screening of Exiqon miRNA qPCR panel chip and subsequent series of qRT-PCR verification. At the same time, 19 pairs of lung adenocarcinoma and adjacent tissues, as well as 5 arterial plasma samples were collected. The selected patients' plasma samples were all from newly diagnosed patients who had not undergone surgery, radiotherapy and chemotherapy intervention and were confirmed as lung adenocarcinoma by pathology. The demographic data and clinical data of these samples were collected systematically.
[0041] Refer to the flowchart ( figure 1 ), randomly selected 30 lung adenocarcinoma samples and 10 n...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com