A kind of method of synthesizing palbociclib intermediate
A synthesis method and a technology for intermediates, applied in the field of organic preparation, can solve the problems of high cost, expensive reagents, and many process steps, and achieve the effects of less impurities and easy control.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 2
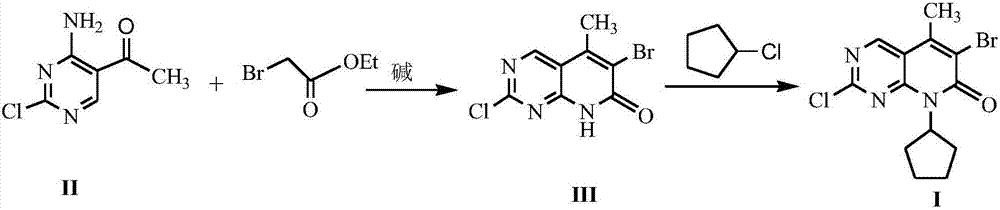
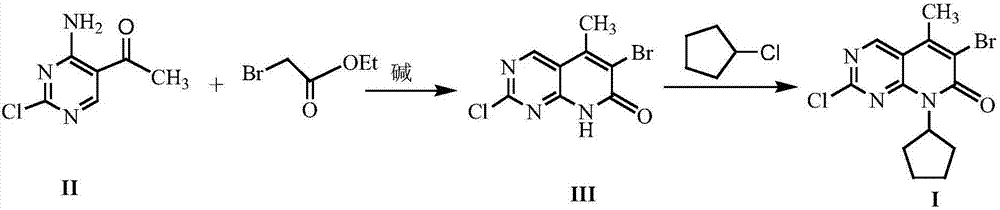
[0019] The preparation of embodiment 2 formula (III) compound 2-chloro-6-bromo-5-methyl-8H-pyrido[2,3-d]pyrimidin-7-one
[0020] Add 10mmol 4-amino-2-chloro-5-pyrimidineethanone, 18mmol ethyl bromoacetate, 20mmol triethylamine and 30mL dry tetrahydrofuran into the reaction flask, stir and mix well. Stir the reaction under reflux for 7 h, stop heating, distill off the solvent under reduced pressure, add 20 mL of 1N HCl to the residue, stir for 1 h and let stand, filter the precipitated solid with suction, dry and recrystallize with isopropanol to obtain formula (III) Compound, yield 74%.
Embodiment 3
[0021] The preparation of embodiment 3 formula (I) compound 2-chloro-6-bromo-8-cyclopentyl-5-methyl-8H-pyrido[2,3-d]pyrimidin-7-one
[0022] With 10mmol formula (III) compound 2-chloro-6-bromo-5-methyl-8H-pyrido[2,3-d]pyrimidin-7-one, 0.6mmol sodium acetate, 15mmol chlorocyclopentane and 30mL Anhydrous ethanol was added into the reaction flask, stirred and mixed evenly, and reacted at room temperature for 7 hours. The solvent was distilled off under reduced pressure, and 50 mL of petroleum ether and 20 mL of 1N HCl were added to the residue. A white solid was precipitated, filtered by suction, dried and recrystallized with isopropanol to obtain the compound of formula (I) with a yield of 88%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com