Quantitative method for occupancy of N-bond sialylated sugar chains on glycoprotein and application of quantitative method in hepatoma marker screening
A technology of sialylation and glycoprotein, applied in measuring devices, material analysis by electromagnetic means, instruments, etc., to achieve the effect of easy-to-obtain materials, easy operation, and high enrichment specificity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] Example 1 Occupancy of N-linked sialylated sugar chains on quantitative standard glycoproteins
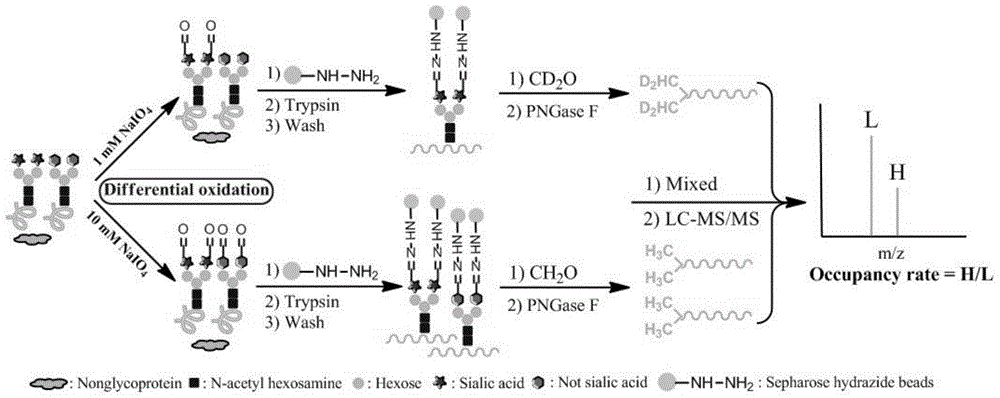
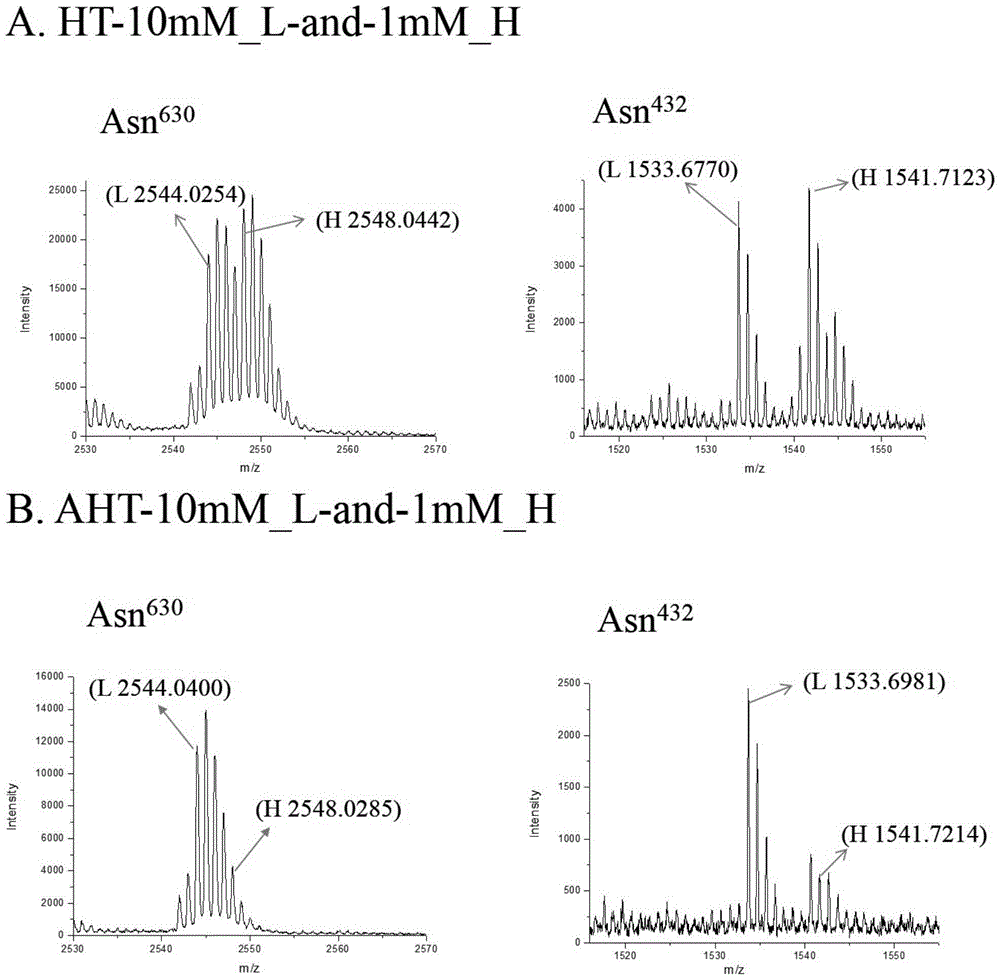
[0026] Human serum transferrin (HT) is a simple and easy-to-obtain glycoprotein, and the sugar chain ends on its two N-glycosylation sites are all sialic acid (document 7.Satomi, Y., Shimonishi, Y .,Hase,T.&Takao,T.Site-specific carbohydrate profiling of human transferrin by nano-flow liquid chromatography / electrospray ionization mass spectrometry.Rapid communications in mass spectrometry:RCM18,2983-2988,doi:10.1002 / rcm.1718( 2004).). The terminal sialic acid can be partially removed by desialidase to obtain desialylated transferrin (AHT) (Reference 6). The experimental process for quantifying the occupancy of N-linked sialylated sugar chains on these two standard glycoproteins using the method described in the present invention is as follows: figure 1 shown.
[0027] 1. Two aliquots of 1mg each of the two standard glycoproteins were dissolved in 100μL containing 8M Urea ...
Embodiment 2
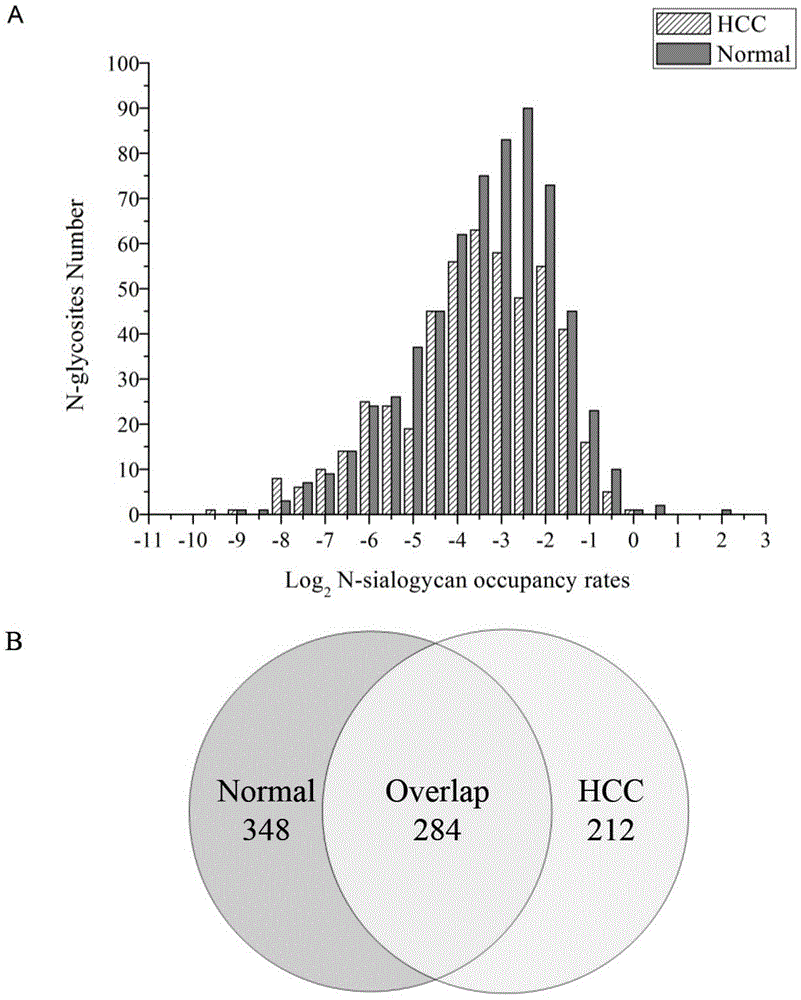
[0037] We used this method to quantify the occupancy of N-linked sialylated sugar chains on glycoproteins in the liver tissues of liver cancer patients and normal people, and compared the N-linked sialylated sugar chains on glycoproteins in the two states Changes in the occupancy rate of HCC, thereby discovering potential biomarkers in human liver cancer.
[0038] The human liver tissue samples used in this experiment were provided by the Second Affiliated Hospital of Dalian Medical University (Dalian, China), which are non-cancerous tissues around the liver (≥ 2 cm) of hepatocellular carcinoma (HCC). The normal tissue part (Normal) had been pathologically sliced, and the acquisition and use of the samples were completely legal and in compliance with the relevant regulations of the ethics committee of the hospital. Protein solutions extracted from liver tissue were processed in the same manner as standard proteins, such as figure 1 shown. Finally, the occupancy rate of 496 s...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com