Use of urea apolipoprotein C-II
A technology of C-II and apolipoprotein, applied in the application field of urine apolipoprotein C-II, can solve the problem of low level of plasma ApoC-II
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0016] Example 1 Urine Specimen Collection and Processing
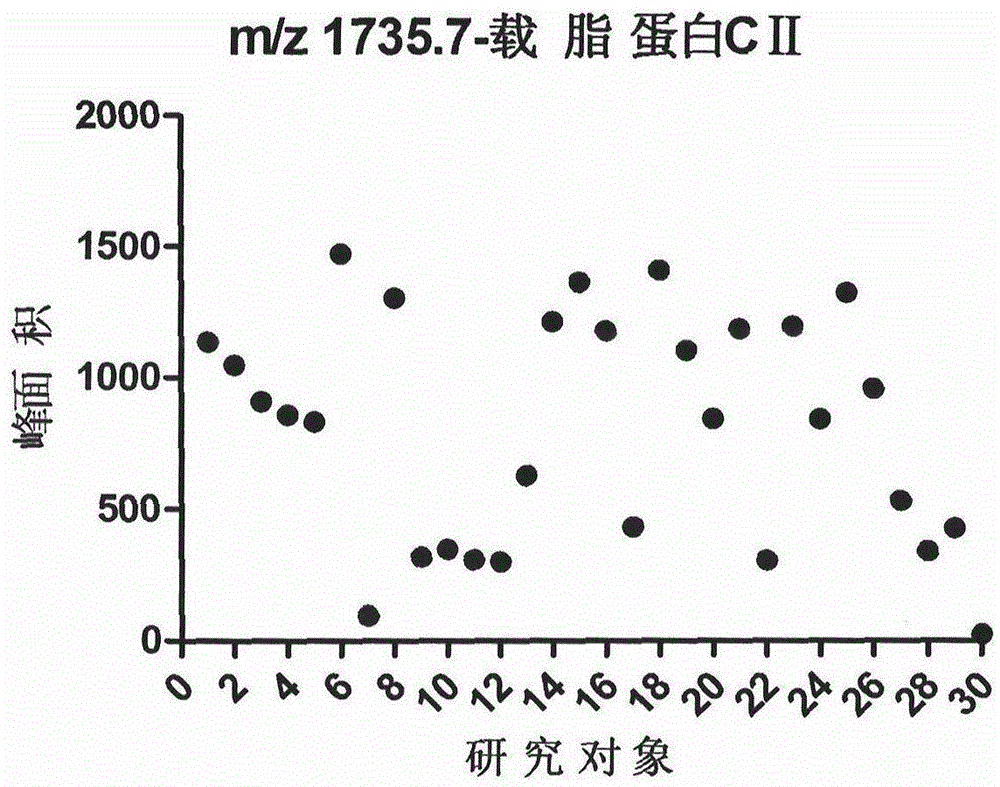
[0017] Collect 30 cases of normal physical examination (Physical Examination Center of Beijing Shijitan Hospital Affiliated to Capital Medical University) and randomly clean midstream urine samples, centrifuge within 2 hours (1500rpm, 5min), and keep the supernatant. Store in a -80°C refrigerator after aliquoting.
Embodiment 2
[0018] Example 2 Magnetic Bead Purification and Isolation of Peptides from Urine Specimens
[0019] Take out the urine sample from the -80°C refrigerator, rethaw at 4°C, centrifuge (3000rpm, 10min), and take the supernatant for later use. Equilibrate Weak Cationic Magnetic Beads (MB-WCX) at room temperature and mix the magnetic bead suspension by hand. Add 10ul of MB-WCX and 10ul of magnetic bead binding buffer into the sample tube, pipette the sample gun up and down to mix well to avoid foaming. Add 5 ul of urine supernatant to the sample tube, mix well and let stand on the magnetic stand for 1 minute to separate the magnetic beads from the suspended liquid. Use a sampling gun to remove the suspended clear liquid, and the tip of the gun should avoid contact with the magnetic beads to avoid absorbing the magnetic beads. Add 100ul of magnetic bead washing buffer into the sample tube, mix well, and then place the sample tube on the magnetic stand for 1 minute, the magnetic ...
Embodiment 3
[0020] Example 3 Spot Targeting and Peptide Spectrum Generation of Urine Specimens
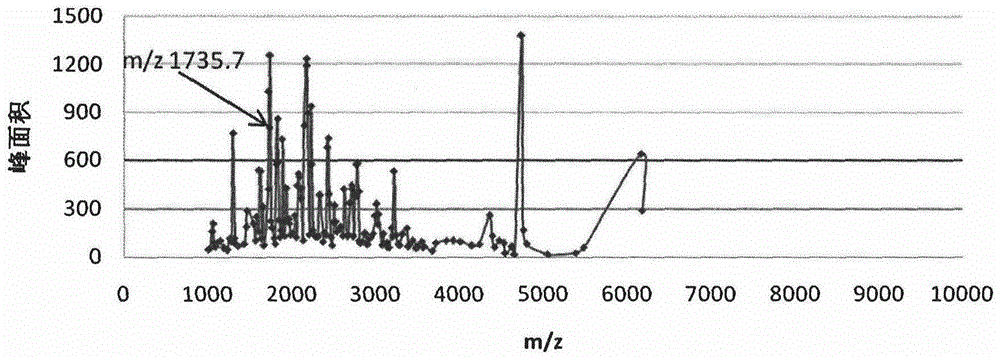
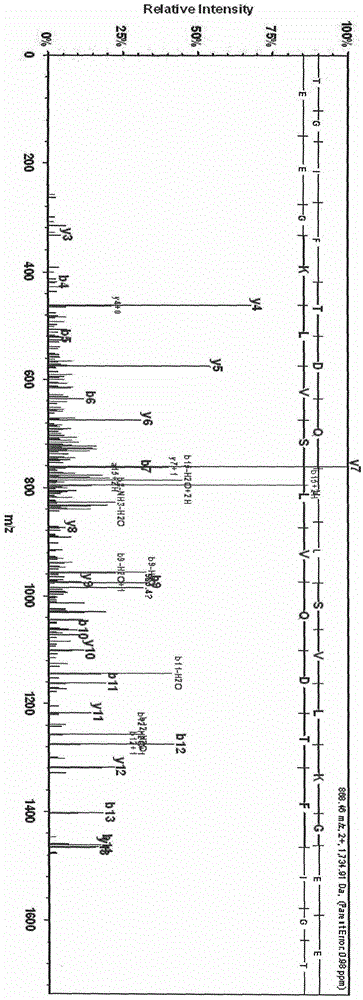
[0021] After calibrating the instrument with a standard, mix 1 μl of eluate with 10 μl of matrix (0.3% α-cyano-4-hydroxycinnamic acid, HCCA), and take 1 μl to spot on the Anchorchip (Autoflex MALDI TOF, Bruker-Dalton) target plate and dry at room temperature. The sample is ionized by nitrogen laser irradiation and then subjected to mass spectrometry analysis, collecting data in the range of 1000-10000 Da, and obtaining a mass spectrogram composed of protein peaks with different mass-to-charge ratios. For each MALDI crystallization point, a total of 400 laser irradiations (50 times for each crystallization point at 8 different positions) were irradiated, and the average value represented one sample, so as to obtain the peptide maps of all samples. ClinProTools2.1 analysis software was used to analyze the mass spectrograms of the normal control group, type 2 diabetes without complications an...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com