Preparation method of 4,5-dicyan base-2-trifluoro-methylimidazole and prepared intermediate and salt thereof
A technology of trifluoromethyl imidazole salt and trifluoromethyl imidazole, which is applied in the field of chemical synthesis, can solve the problems of low boiling point, volatile measurement, inconvenience of use, etc. , the effect of simple process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
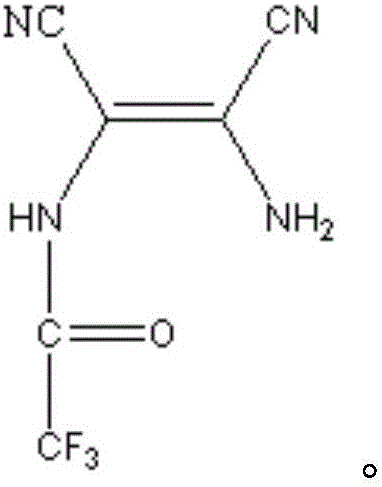
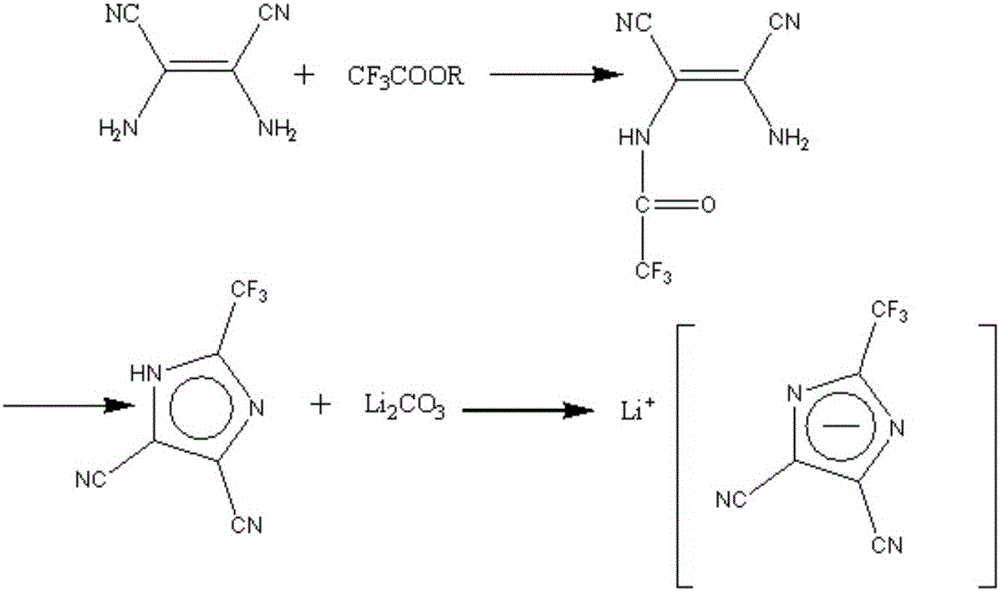
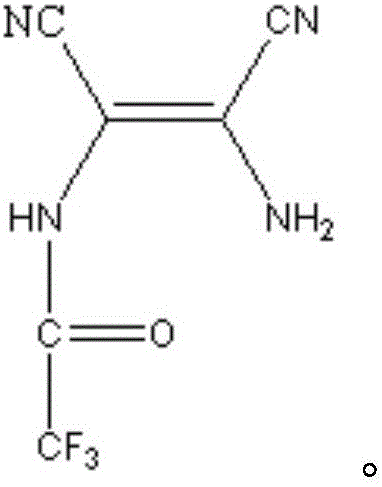
[0047] In a 1L four-necked round-bottom flask equipped with an electric stirrer, reflux condenser, thermometer, and gas guide tube, add 43.24 g (0.4 mol) of diaminomaleonitrile and 51.22 g of methyl trifluoroacetate in a glove box at room temperature (0.4mol), 1,4-dioxane 500mL, set up the device, turn on the ventilation equipment to ensure that there is dry argon protection throughout the reaction. Stir at 25°C for 2 hours to fully react the two reactants to form an amide, and then raise the temperature to the boiling point of 1,4-dioxane (101°C) to ensure the reflux of the system. At this temperature, the amide dehydrates to form imidazole, and stops after 2 hours of reflux After the heating reaction, a mixed solution containing 4,5-dicyano-2-trifluoromethylimidazole was obtained.
[0048] Evaporate the solvent and residual methyl trifluoroacetate under the protection of dry argon, add 600ml of water to the system, add 24g of activated carbon to remove color, heat slightly b...
Embodiment 2
[0051] In a 250mL four-necked round-bottom flask equipped with an electric stirrer, reflux condenser, thermometer, and gas guide tube, add 43.24g (0.4mol) of diaminomalenitrile and 68.05g of butyl trifluoroacetate in a glove box at room temperature (0.4mol), set up the device, turn on the ventilation equipment to ensure that there is dry argon protection throughout the reaction. Heating to 100°C, the boiling point of butyl trifluoroacetate, facilitates the reflux of the system. At this temperature, the two reactants first form an amide, and then the amide is dehydrated to form an imidazole ring. The reaction lasts for 3 hours. After the reaction, stop heating and wait for the reaction system to cool. A mixed solution containing 4,5-dicyano-2-trifluoromethylimidazole can be obtained.
[0052] Transfer the mixed solution containing 4,5-dicyano-2-trifluoromethylimidazole into a 1L flask, add 600ml of water, add 24g of activated carbon to remove color, and heat it slightly before ...
Embodiment 3
[0055] In a 1L four-necked round-bottom flask equipped with an electric stirrer, reflux condenser, thermometer, and gas guide tube, add 43.24 g (0.4 mol) of diaminomalenitrile and 142.08 g of ethyl trifluoroacetate in a glove box at room temperature (1mol), 1,4-dioxane 500mL, set up the device, turn on the ventilation equipment to ensure that there is dry argon protection throughout the reaction. Stir at 50°C for 2 hours to fully react the two reactants to form an amide, and then raise the temperature to the boiling point of 1,4-dioxane (101°C) to ensure the reflux of the system. At this temperature, the amide dehydrates to form imidazole, and stops after 2 hours of reflux After the heating reaction, a mixed solution containing 4,5-dicyano-2-trifluoromethylimidazole was obtained.
[0056] Evaporate and remove the solvent and residual ethyl trifluoroacetate under the protection of dry argon, add 600ml of water to the system, add 24g of activated carbon to remove the color, and ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com