Fluorene and carbazole bridging-based A-D-A type double-center BODIPY (boron-dipyrrolemethene) derivative and preparation method for same
A technology of A-D-A and dipyrrole methine is applied in the field of organic optoelectronic materials, which can solve the problems of single molecular structure, not many reports, and few samples.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
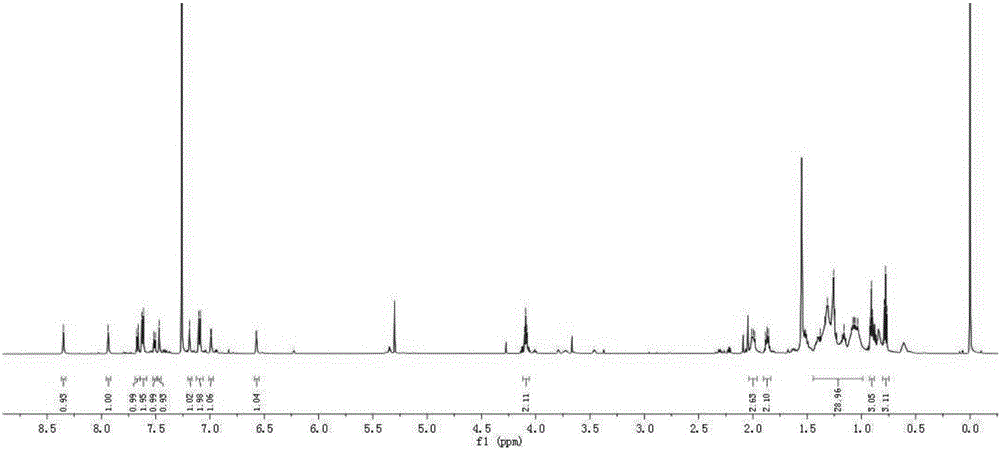
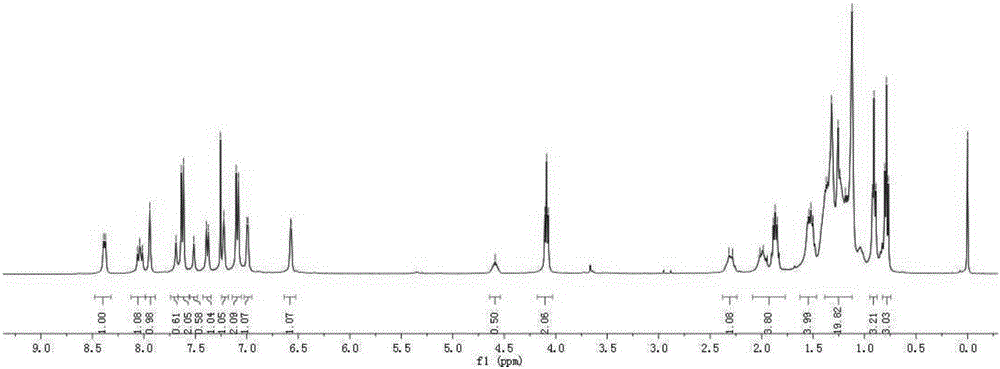
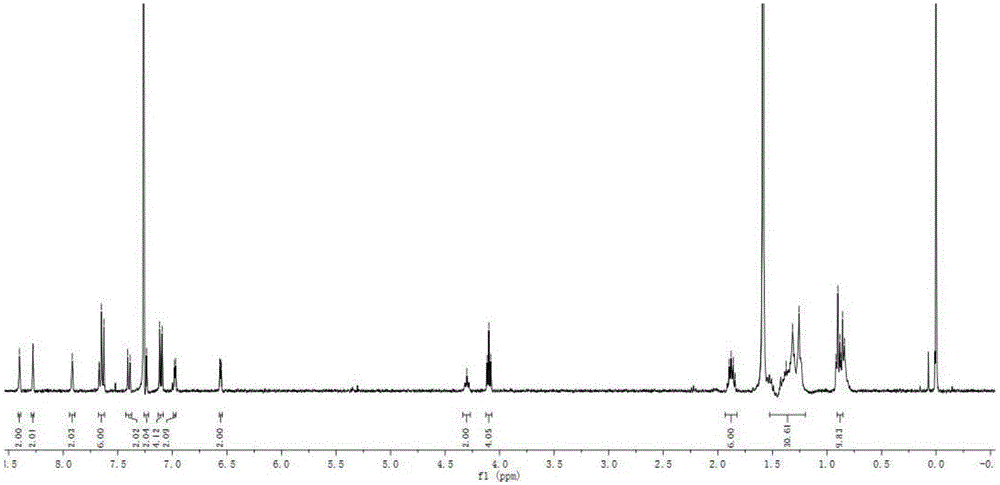
[0048] (1) Synthesis of Intermediate 1
[0049] Add p-hydroxybenzaldehyde (12.5g, 0.1mol), potassium carbonate (16.5g, 0.12mol), bromo-n-octane (23.2g, 0.12mol) and acetonitrile (150mL) successively in a 500mL round bottom flask, The reaction temperature was controlled at 80° C. for 10 h with magnetic stirring. After the reaction was completed, the solid residue was removed by filtration with a sand core funnel, the filtrate was extracted with ethyl acetate, washed with saturated brine, and dried with anhydrous magnesium sulfate to obtain a crude product. The crude product was purified by silica gel (200-300 mesh) column chromatography [eluent, V (petroleum ether): V (ethyl acetate) = 10:1] to obtain yellow liquid intermediate 1, 22.2 g, yield 95%. 1 H NMR (600MHz, CDCl 3 ), δ: 9.82 (d, J = 1.4Hz, 1H), 7.77 (dd, J = 8.7, 1.5Hz, 2H), 6.93 (dd, J = 8.7, 1.5Hz, 2H), 3.97 (td, J = 6.6,1.6Hz,2H),1.82-1.65(m,2H),1.50-1.35(m,2H),1.35-1.18(m,10H),0.87(m,3H). 13 C NMR (151MHz, CDC...
PUM
| Property | Measurement | Unit |
|---|---|---|
| energy conversion efficiency | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com