D-pai-A-pai-D type BODIPY (boron-dipyrromethene) derivatives based on acetenyl bridging and preparation method of D-pai-A-pai-D type BODIPY derivatives
An ethynyl and derivative technology, applied in the field of organic small molecule solar cell materials, can solve the problems of lack of sufficient molecular design and synthesis route optimization, low photovoltaic efficiency, single structure, etc., and achieves easy control of synthesis reaction conditions and wide ultraviolet absorption. Scope, effect of novel molecular structures
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0100] Synthesis of target molecule BDP1
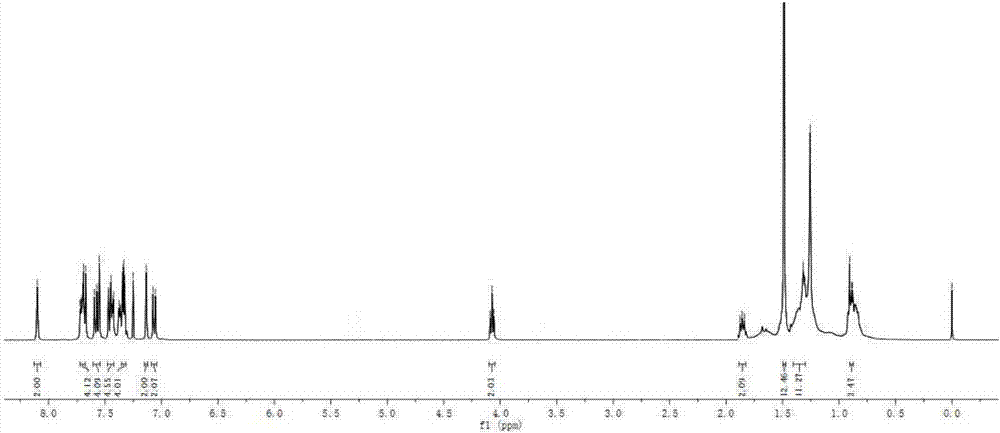
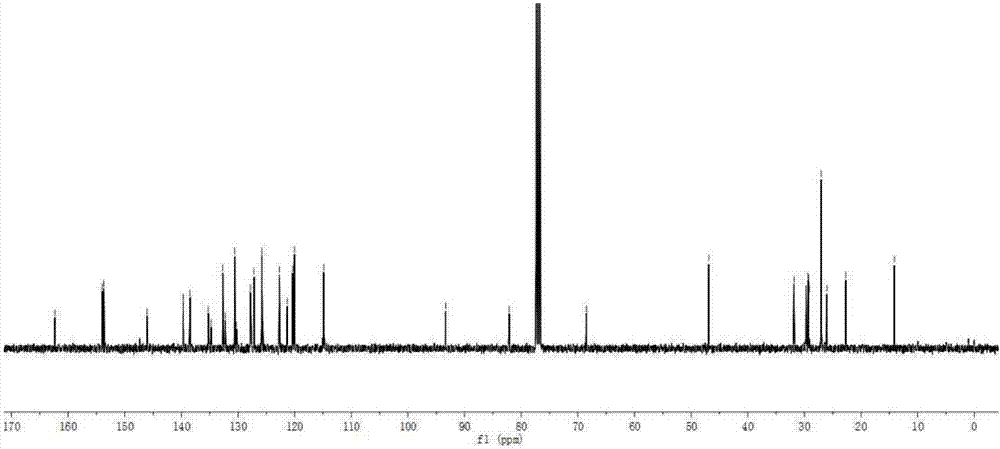
[0101] Add intermediate 3 (65mg, 0.1mmol), intermediate 4 (55mg, 0.25mmol), CuI (2mg, 0.01mmol), PdCl 2 (PPh 3 ) 2 (7mg, 0.01mmol), toluene (10mL) and triethylamine (10mL), vacuumize, pass through argon protection, and react with magnetic stirring at room temperature for 12h. Stop the reaction, extract with ethyl acetate, wash with saturated brine, and dry over anhydrous magnesium sulfate. Filtered, and the filtrate was rotovapped to remove the solvent. The crude product was purified by silica gel (200-300 mesh) column chromatography [eluent, V (petroleum ether): V (ethyl acetate) = 10:1] to obtain dark green solid BDP1 (59 mg), yield 71%. 1 H NMR (400MHz, CDCl 3 )δ:8.10(s,2H),7.70(dd,J=6.5,2.6Hz,4H),7.61–7.54(m,4H),7.48–7.42(m,4H),7.34(dd,J=6.0, 2.7Hz, 4H), 7.13(s, 2H), 7.06(d, J=8.7Hz, 2H), 4.07(t, J=6.5Hz, 2H), 1.89–1.83(m, 2H), 1.49(s, 12H), 1.31(d, J=6.6Hz, 10H), 0.88(d, J=3.6Hz, 3H). 13 C NMR (101MHz, CDCl 3 )δ:162.33,1...
Embodiment 2
[0103] Synthesis of target molecule BDP2
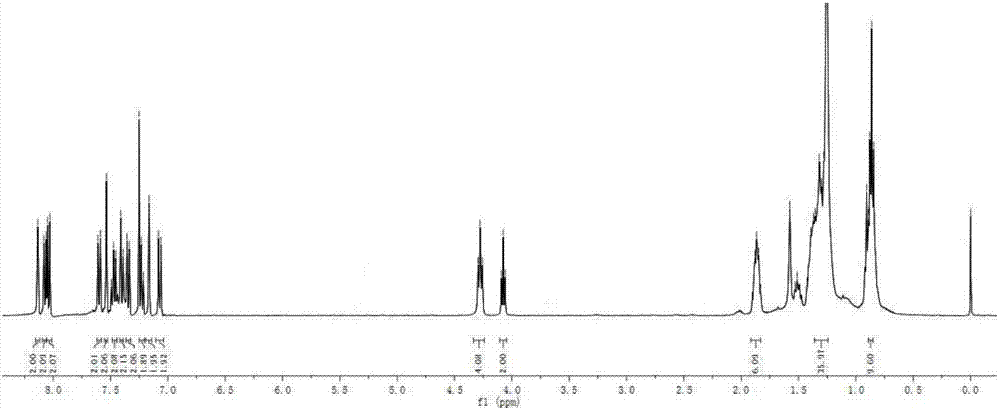
[0104] The synthesis method of BDP2 was similar to the synthesis method of BDP1. Intermediate 3 (65 mg, 0.1 mmol) and intermediate 5 (76 mg, 0.25 mmol) were used as substrates to obtain dark green solid BDP2 (68 mg) with a yield of 68%. 1 H NMR (400MHz, CDCl 3 )δ:8.13(s,2H),8.07(d,J=7.7Hz,2H),8.04(d,J=8.0Hz,2H),7.60(d,J=8.6Hz,2H),7.54(s, 2H), 7.46(d, J=7.2Hz, 2H), 7.40(d, J=8.2Hz, 2H), 7.35(d, J=8.0Hz, 2H), 7.22(d, J=7.3Hz, 2H) ,7.16(s,2H),7.07(d,J=8.7Hz,2H),4.28(t,J=7.2Hz,4H),4.08(t,J=6.5Hz,2H),1.92–1.83(m, 6H),1.36–1.24(m,30H),0.89–0.85(m,9H). 13 C NMR (101MHz, CDCl 3 )δ:162.31,146.11,141.18,140.00,134.83,132.69,132.20,126.25,125.68,123.10,122.48,122.33,120.61,120.34,119.49,119.18,115.07,114.84,113.20,111.81,108.87,94.01,81.57,68.50 ,43.22,31.85,31.82,29.73,29.40,29.27,29.20,29.15,28.98,27.33,26.06,22.70,22.63,14.14,14.09. 67 h 73 BF 2 N 4 O[M] + :998.585; found 998.294.
Embodiment 3
[0106] Synthesis of target molecule BDP3
[0107]The synthesis method of BDP3 is similar to the synthesis method of BDP1, using intermediate 3 (65mg, 0.1mmol) and intermediate 6 (124mg, 0.25mmol) as substrates, the dark green solid BDP2 (95mg) was purified with a yield of 69%. 1 H NMR (400MHz, CDCl 3 )δ: 8.03(s, 2H), 7.56(d, J=8.5Hz, 2H), 7.26(dd, J=8.5, 5.5Hz, 12H), 7.04(d, J=8.6Hz, 4H), 7.01( s,4H),6.99(s,4H),6.94(s,2H),6.92(s,2H),4.06(t,J=6.5Hz,2H),1.88–1.81(m,2H),1.71(s ,8H),1.36(m,34H),0.75(m,39H). 13 C NMR (101MHz, CDCl 3 )δ:162.32,148.41,146.00,145.48,144.20,132.61,132.27,127.12,125.74,125.71,124.39,121.23,114.78,114.51,93.03,81.01,68.45,57.17,38.26,32.44,31.84,31.75,31.44,29.72 , 29.34, 29.26, 29.14, 26.04, 22.69, 14.13. MALDI-TOF-MS, m / z: calcd for C 95 h 117 BF 2 N 4 O[M] + :1378.929; found 1378.320.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com