Meso-ethynyl bridged D-Pi-A type BODIPY dye and preparation method thereof
An ethynyl and bridging technology, which is applied in the direction of methynyl/polymethynyl dyes, organic dyes, chemical instruments and methods, etc., can solve problems such as difficult to obtain, and achieve easy control of reaction conditions, universal applicability, and product Purify Simple Effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0079] Synthesis of BDP1:
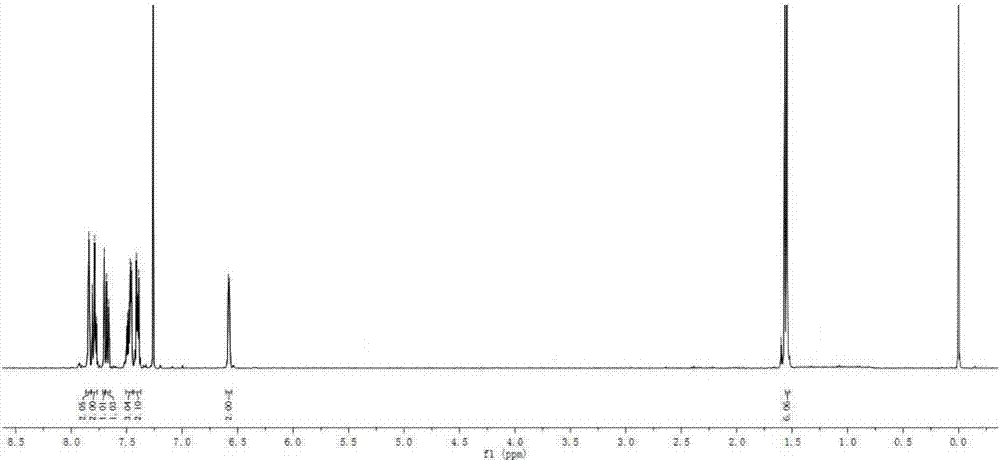
[0080] Add intermediate 3 (226mg, 1.0mmol), intermediate 4 (262mg, 1.2mmol), anhydrous THF (20mL) and anhydrous Et to a 50mL three-neck flask in sequence 3 N (2mL), the system was evacuated, protected by argon, the reaction bottle was placed in an ice-water bath, and CuI (10mg, 0.05mmol) and Pd(PPh 3 ) 2 Cl 2 (35mg, 0.05mmol), stirred magnetically at 0°C for 30min. Stop the reaction, pour into 50mL distilled water, extract with ethyl acetate (30mL×3), wash the organic phase with saturated brine (30mL×3), anhydrous Na 2 SO 4 Let dry overnight. The filtrate was collected by filtration, the solvent was evaporated under reduced pressure, and the residue was purified by silica gel (200-300 mesh) column chromatography [eluent, V (petroleum ether): V (ethyl acetate) = 16:1] to obtain a red solid BDP1 (290 mg), yield 71%. 1 H NMR (400MHz, CDCl 3 )δ:7.84(s,2H),7.82–7.77(m,2H),7.70(d,J=0.7Hz,1H),7.67(dd,J=7.8,1.4Hz,1H),7.51–7.45(m ,3H),7.44–7.37(m,2H...
Embodiment 2
[0082] Synthesis of BDP2:
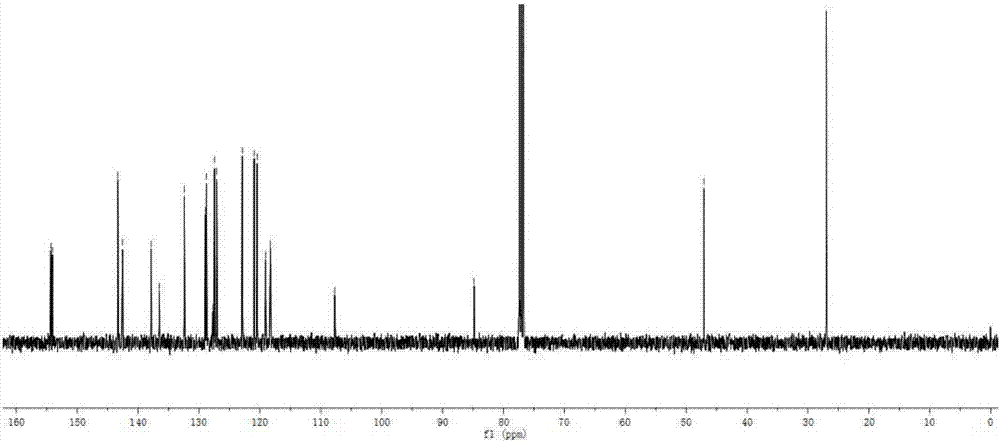
[0083] The synthesis method of BDP2 was similar to the synthesis method of BDP1. Intermediate 3 (226mg, 1.0mmol) and intermediate 5 (564mg, 1.2mmol) were used as substrates to obtain dark red solid BDP2 (390mg) with a yield of 59%. 1 H NMR (400MHz, CDCl 3 )δ:7.94(s,1H),7.85(s,2H),7.49(q,J=5.6Hz,2H),7.43(d,J=4.0Hz,2H),6.59(d,J=3.3Hz, 2H), 4.35(t, J=6.6Hz, 2H), 4.28(t, J=6.6Hz, 2H), 1.96–1.86(m, 4H), 1.35(d, J=25.9Hz, 20H), 0.90( dd,J=6.7,4.7Hz,6H). 13 C NMR (101MHz, CDCl 3 )δ:145.67,143.90,143.68,136.16,134.22,131.02,130.62,130.46,130.40,128.98,128.15,126.37,120.44,119.62,118.51,99.68,90.05,74.42,74.18,31.88,30.56,29.45,29.34,26.08 , 22.73, 14.19. MALDI-TOF-MS, m / z: calcd for C 37 h 43 BF 2 N 2 o 2 S 2 [M] + :660.283,found:660.183.
Embodiment 3
[0085] Synthesis of BDP3:
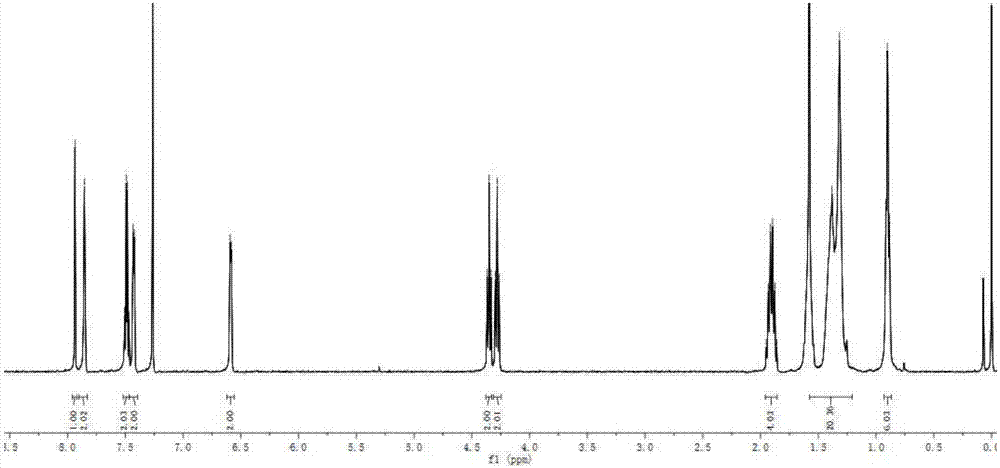
[0086]The synthesis method of BDP3 was similar to the synthesis method of BDP1. Intermediate 3 (226 mg, 1.0 mmol) and intermediate 6 (402 mg, 1.2 mmol) were used as substrates to obtain BDP3 (326 mg) as a purple-black solid with a yield of 62%. 1 H NMR (400MHz, CDCl 3 )δ: 7.81(s, 2H), 7.45(dd, J=8.5, 1.9Hz, 1H), 7.39–7.34(m, 3H), 7.18(dd, J=11.1, 4.5Hz, 1H), 7.12(dd ,J=7.6,1.5Hz,1H),6.97(t,J=7.0Hz,1H),6.87(dd,J=14.6,8.2Hz,2H),6.55(d,J=2.8Hz,2H),3.92 –3.85(m, 2H), 1.81(dd, J=14.9, 7.6Hz, 2H), 1.56–1.18(m, 10H), 0.87(t, J=6.8Hz, 3H). 13 C NMR (101MHz, CDCl 3 )δ:148.06,143.63,142.77,136.21,132.82,131.28,128.51,127.76,127.65,127.57,125.05,123.49,118.04,115.93,115.12,114.05,107.56,107.52,85.76,47.88,31.76,29.23,29.19,26.85 , 26.76, 22.66, 14.15. MALDI-TOF-MS, m / z: calcd for C 31 h 30 BF 2 N 3 S[M] + :525.222,found:525.203.
[0087] The photophysical and thermal stability related data of the target dye BDP1-3 in the above examples are shown in T...
PUM
| Property | Measurement | Unit |
|---|---|---|
| thermal decomposition temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com