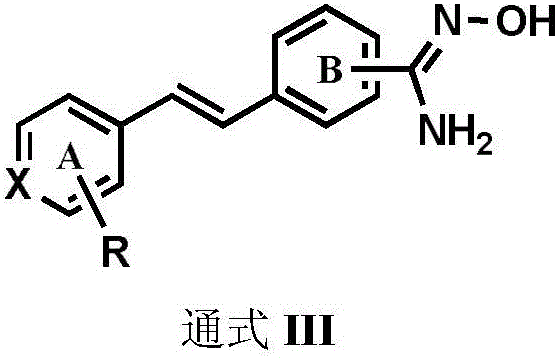
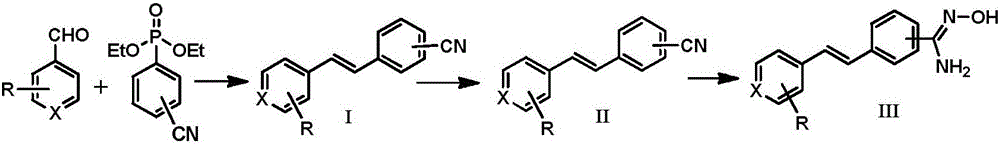
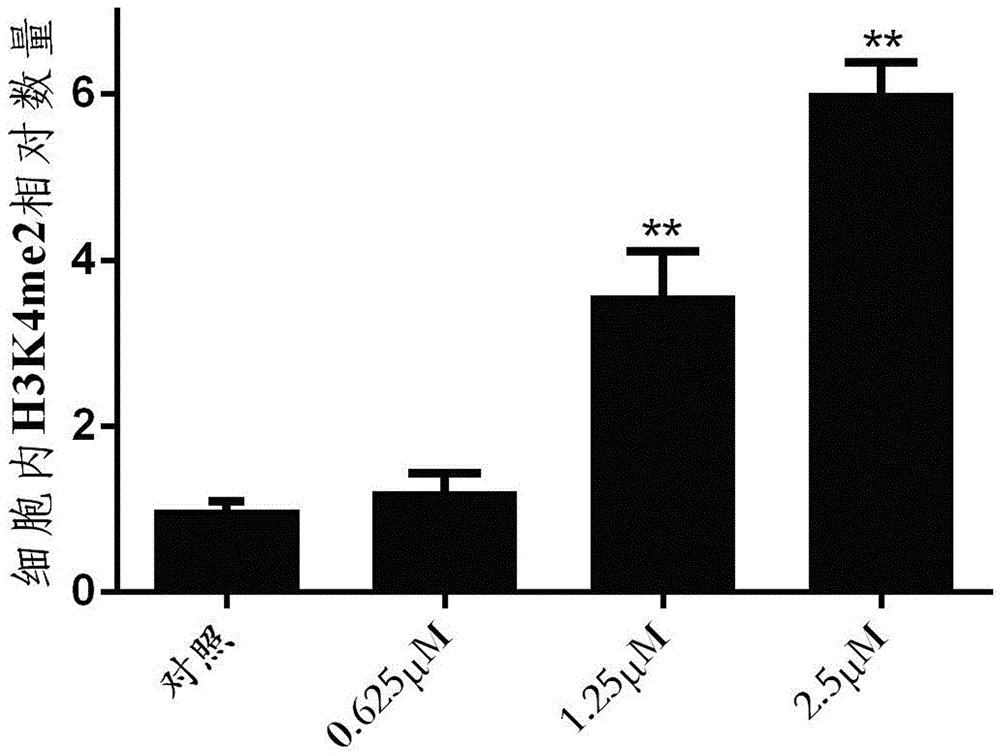Resveratrol derivative, preparation method thereof and application of resveratrol derivative serving as LSD1 inhibitor
A technology of resveratrol and derivatives, applied in antiviral agents, drug combinations, organic chemistry, etc., to achieve strong LSD1 inhibitory activity, high yield, and favorable effects for popularization and application
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] The preparation of embodiment 1 (E)-4-(3,4-dimethoxystyryl) benzyl cyanide (I-1)
[0037]
[0038] The compound 3,4-dimethoxybenzaldehyde (1.66g, 10mmol) and diethyl 4-cyanobenzylphosphonate (2.79g, 11mmol) were dissolved in anhydrous DMF (10mL), stirred in an ice bath Slowly add potassium tert-butoxide (2.24g, 20mmol), and react at room temperature for 3 hours after the addition, slowly add the reaction system into ice water (40mL), a white solid is washed out, suction filtered, washed with water, the solid is collected, and washed with acetone Recrystallized, filtered with suction, and dried in vacuo to obtain 2.21 g of a white solid with a yield of 83.4%. Mp: 102-103°C. 1 H NMR (400MHz, CDCl 3 )δ7.64(d, 2H, J=8.0Hz), 7.58(d, 2H, J=8.0Hz), 7.19(d, 1H, J=16.4Hz), 7.10(m, 2H), 6.98(d, 1H,J=16.4Hz),6.90(d,1H,J=8.0Hz),3.97(s,3H),3.93(s,3H). 13 CNMR (101MHz, CDCl 3 )δ149.76, 149.22, 142.11, 132.48, 132.22, 129.36, 126.59, 124.74, 120.76, 119.17, 111.19, 110.10, 108...
Embodiment 2
[0039] The preparation of embodiment 2 (E)-4-(2-fluoro-4,5-dimethoxystyryl) benzyl cyanide (I-2)
[0040]
[0041] According to the method of Example 1, 2-fluoro-4,5-dimethoxybenzaldehyde (1.84g, 10mmol) was used to replace 3,4-methoxybenzaldehyde to obtain 2.12g of white solid with a yield of 74.9%. Mp: 130-131°C. 1 H NMR (400MHz, CDCl 3 )δ7.66(d, 2H, J=8.0Hz), 7.61(d, 2H, J=8.0Hz), 7.36(d, 1H, J=16.0Hz), 7.06(d, 1H, J=8.0Hz) ,7.04(d,1H,J=16.0Hz),6.69(d,1H,J=12.0Hz),3.95(s,3H),3.92(s,3H). 13 C NMR (101MHz, CDCl 3 )δ156.68,154.24,150.50,150.40,145.64,145.62,142.00,132.51,126.37,126.32,124.47,124.44,119.09,115.29,115.16,110.44,108.35,108.30,100.30,100.02,56.46,56.24.HRMS(ESI)calcd for C 17 h 15 FNO 2 [M+H] + :284.1081,Found:284.1081.
Embodiment 3
[0042] The preparation of embodiment 3 (E)-4-(2-bromo-4,5-dimethoxystyryl) benzyl cyanide (I-3)
[0043]
[0044] According to the method of Example 1, 2-bromo-4,5-dimethoxybenzaldehyde (1.23g, 5mmol) was used to replace 3,4-dimethoxybenzaldehyde to obtain 1.31g of white solid, yield 76.3% . Mp: 149-151°C. 1 H NMR (400MHz, CDCl 3 )δ7.67(d, 2H, J=8.0Hz), 7.63(d, 2H, J=8.0Hz), 7.55(d, 1H, J=16.0Hz), 7.16(s, 1H), 7.08(s, 1H), 6.95(d, 1H, J=16.0Hz), 3.97(s, 3H), 3.92(s, 3H). 13 C NMR (101MHz, CDCl 3 )δ150.10, 148.74, 141.70, 132.55, 130.93, 128.19, 127.31, 126.97, 119.04, 115.74, 115.54, 110.69, 108.68, 56.25, 56.16. HRMS (ESI) calcd for C 17 h 14 BrNNaO 2 [M+Na] + :366.0106,Found:366.0101.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com