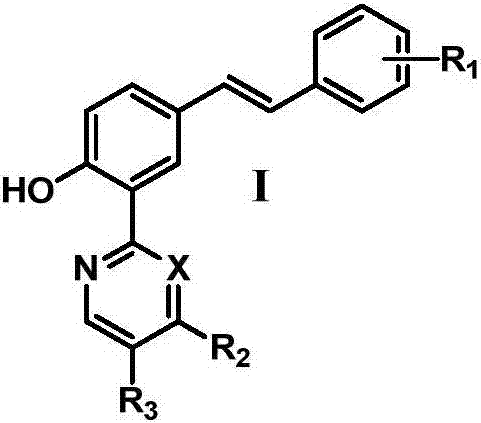
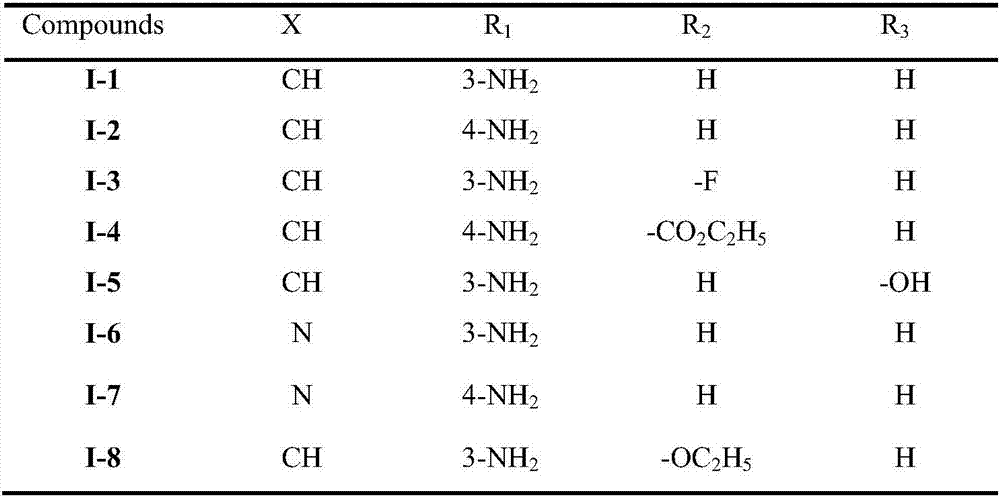
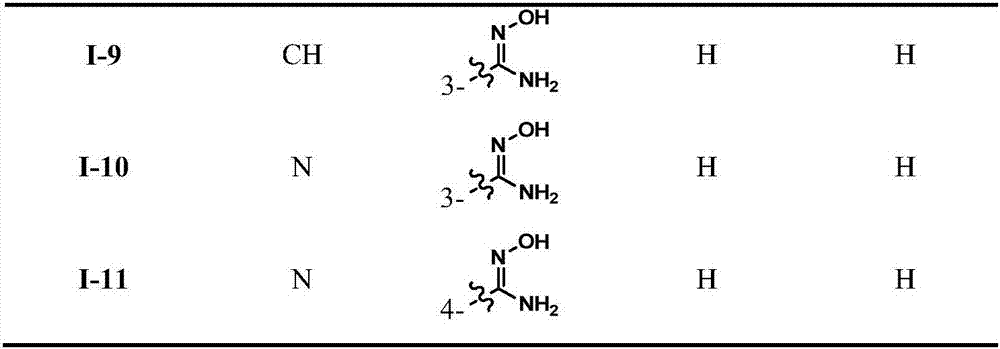
Trans-diaryl ethylene LSD1 (lysine specific histone demethylase 1) inhibitor, as well as preparation method and application thereof
A diarylethene, reaction technology, applied in the directions of organic chemistry, drug combination, anti-tumor drugs, etc., achieves strong in vitro anti-tumor activity, strong LSD1 inhibitory activity, and is conducive to popularization and application.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] Example 1 4-methoxy-3-(pyridin-2-yl)benzaldehyde (1a)
[0027]
[0028] Add 2-bromopyridine (456.5mg, 2.89mmol), toluene (7mL), K 2 CO 3 Aqueous solution (2.76g potassium carbonate dissolved in 10mL water, 2mL), tetrakis(triphenylphosphine)palladium (45.2mg, 0.04mmol), stirred at room temperature under nitrogen protection for 15 minutes, then added 5-formyl-2-methoxy Phenylboronic acid (400mg, 2.22mmol) in absolute ethanol (3mL), heated at 92°C for 4 hours, poured the reaction system into water, extracted with ethyl acetate, combined the ethyl acetate layers, followed by water, saturated saline Washed, dried over anhydrous sodium sulfate, suction filtered, the filtrate was concentrated under reduced pressure, and the concentrate was separated by column chromatography (petroleum ether: ethyl acetate = 6:1) to obtain 414.6 mg of white solid, yield 87.5%, Mp: 52-53 ℃. 1 H NMR (400MHz, CDCl 3 )δ9.98(s,1H),8.73(m,1H),8.31(d,1H,J=2.4Hz),7.96(dd,1H,J 1 = 2.4 Hz,J 2 =8...
Embodiment 2
[0029] Example 2 4-methoxy-3-(4-fluoropyridin-2-yl)benzaldehyde (1b)
[0030]
[0031] According to the method of Example 1, 2-bromo-4-fluoropyridine (508.6mg, 2.89mmol) was used instead of 2-bromopyridine to obtain 395.8mg of white solid, yield 77.1%, Mp: 73-74°C.1 H NMR (400MHz, CDCl 3 )δ 10.00(s,1H),8.72(dd,1H,J 1 =5.6Hz,J 2 =8.8Hz),8.40(t,1H,J=2.0Hz),8.00(dd,1H,J 1 =1.2Hz,J 2 =8.8Hz),7.65(d,1H,J=10.4Hz),7.16(d,1H,J=8.4Hz),7.05(m,1H),4.01(s,3H). 13 C NMR (101MHz, CDCl 3 )δ190.88,175.95,169.87,167.27, 161.69,157.37,157.29,151.61,151.54,134.23,131.67,130.04,128.13,128.10, 113.06,112.87,111.74,110.42,110.25,56.11.HRMS(ESI)calcd for C 13 h 10 FNNaO 2 [M+Na] + :254.0588,Found:254.0582.
Embodiment 3
[0032] Example 3 4-methoxy-3-(pyrimidin-2-yl)benzaldehyde (1c)
[0033]
[0034] According to the method of Example 1, 2-bromopyridine was replaced with 2-bromopyrimidine (445.1 mg, 2.80 mmol) to obtain 414.2 mg of white solid, yield 87.1%, Mp: 80-81°C. 1 H NMR (400MHz, CDCl 3 )δ 9.96(s,1H),8.88(d,2H,J=4.8Hz),8.26(d,1H,J=2.4Hz),8.00(dd,1H,J 1 = 2.4 Hz,J 2 =8.8Hz),7.29(t,1H,J=4.8Hz),7.16(d,1H,J=8.8Hz),3.96(s,3H). 13 C NMR (101MHz, CDCl 3 )δ190.61, 164.71, 162.41, 157.17, 134.47, 132.52, 129.64, 128.77, 119.23, 112.05, 56.44. HRMS (ESI) calcdfor C 12 h 10 N 2 NaO 2 [M+Na] + : 237.0634,Found: 237.0639.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com