Novel methods for preparing silodosin and intermediates thereof
A technology of silodosin and intermediates, applied in the field of medicinal chemistry, can solve the problems of long reaction cycle, cumbersome operation, and low product purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0075] Example 1, tert-butyl-(R)-1-(1-(3-(benzoyloxy)propyl)-7-cyanindoline-5-yl)propyl-2-yl- Carbonate (Compound V) Preparation
[0076]
[0077] 500g 5-[(2R)-2-aminopropyl]-2,3-dihydro-1-[3-(benzoyloxy)propyl]-1H-indole-7-carbonitrile tartrate ( Compound SM1) was added to 3.0L of dichloromethane and stirred, then an aqueous solution (4.0L) of potassium carbonate (1.105kg) was added, and 221g (Boc) 2 O, after the dropwise addition, react at 15~25°C for 5 hours, let stand for liquid separation, wash the dichloromethane phase with 3L of 0.2mol / L HCl solution, 2L of saturated sodium bicarbonate solution, and 2L of saturated brine successively, and then Dry over sodium sulfate, filter, and distill off the solvent from the filtrate under reduced pressure to obtain 457g of compound V, with an HPLC purity of 99.57%;
[0078] MS[M+H] + :464;
[0079] 1 H NMR (400 MHz, DMSO): 8.01-7.99(d,2H), 7.67-7.63(m,1H), 7.52-7.49(m,2H), 7.04(s,1H), 6.95(s,1H), 6.72-6.70(m,1H), 4.37(m,2H...
Embodiment 2
[0080] Example 2, tert-butyl (R)-1-(7-carbonamido-1-(3-hydroxypropyl)indoline-5-yl)propyl-2-yl-carbonate (compound IV) preparation of
[0081]
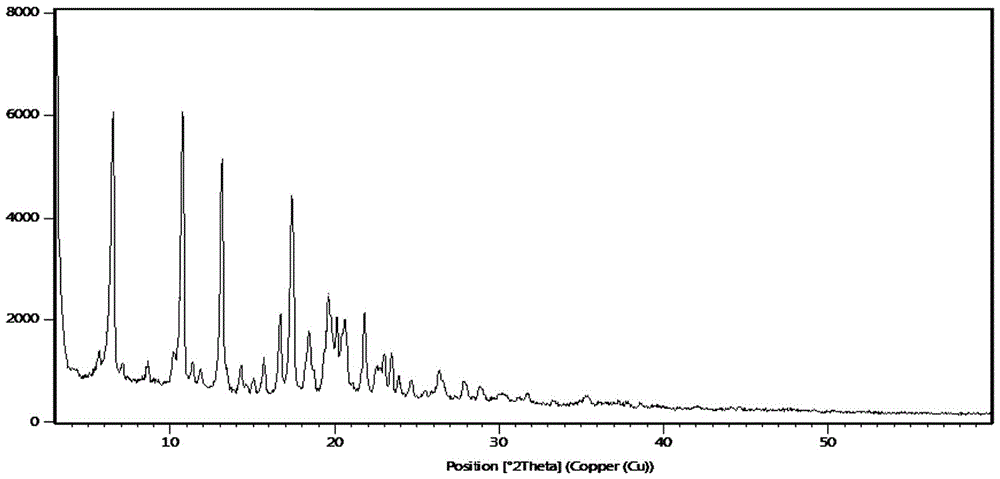
[0082] Add 178g (0.384mol) of compound V to 1.78L of dimethyl sulfoxide and stir to dissolve, control the temperature at 20~25°C, add dropwise 0.168L (0.84mol) of KOH solution with a concentration of 5mol / L, and dropwise add 217g (1.91 mol) with a mass fraction of 30% H 2 o 2 Aqueous solution, reacted at 15~25°C for 5 hours, added 5.34L 5% sodium sulfite aqueous solution to the reaction solution, stirred to precipitate solid, filtered, the filtrate was extracted with ethyl acetate, washed, dried, and the solvent was distilled off under reduced pressure to obtain 119g of compound IV. The yield is 81.5%, the HPLC purity is 97.43%, and the content of the dehydrogenation impurity Q1 is 1.83%. After determination, its X-RPD spectrum is as follows: figure 1 shown;
[0083] MS[M+H] + :378;
[0084] 1 H NMR (400 MHz, DMSO): 7.64(s,1...
Embodiment 3
[0085] Example 3, Preparation of 5-((R)-2-aminopropyl)-1-(3-hydroxypropyl)indoline-7-carbonamide hydrochloride (compound III)
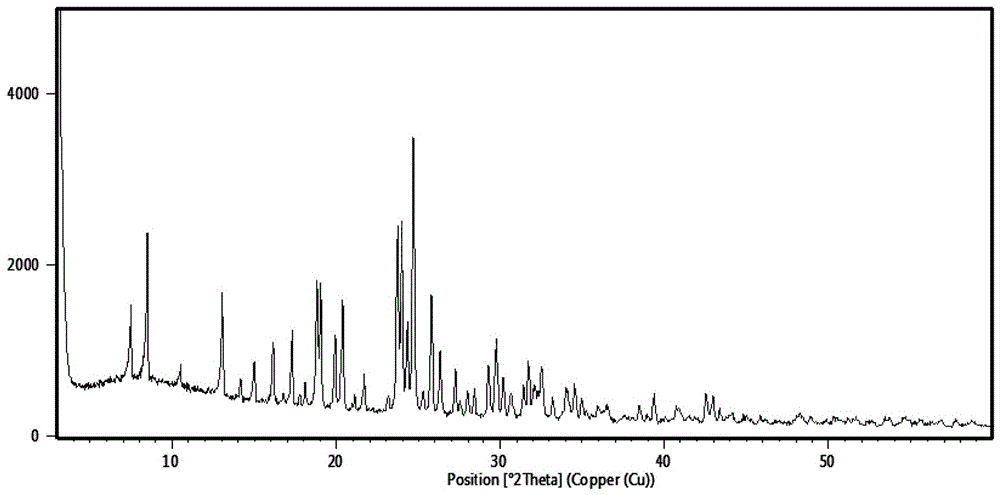
[0086] Add 30g (54.04 mmol) of compound IV into 300mL of ethanol, stir and cool down to 0~10°C, add dropwise 36.2mL of 2mol / L HCl ethanol solution (72.4 mmol), stir at 15~25°C for 5~6h, filter, Dry, obtain 23.8g compound III, HPLC purity 99.59%, the content of dehydrogenation impurity Q2 is 0.18%, after measuring, its X-RPD collection of illustrative plates is as follows figure 2 shown;
[0087] MS[M-Cl] + :278;
[0088] 1 H NMR (400 MHz, DMSO): 8.04(m,2H), 7.74(s,1H), 7.35(s,1H), 6.94-6.89(d,2H), 4.43(br,1H), 3.42-3.16( m,7H); 2.91-2.50(m,4H), 1.62(m,2H),112-1.10(m,3H).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com