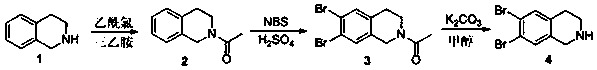A novel compound 6,7-dibromo-1,2,3,4-tetrahydroisoquinoline and its preparation method
A technology of tetrahydroisoquinoline and compound, which is applied in the field of novel compound 6,7-dibromo-1,2,3,4-tetrahydroisoquinoline and its preparation, can solve the problem of expensive raw materials, long steps, and high cost. problems such as low rate, to achieve the effect of easy processing and purification, mild reaction
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0019] The preparation method of 6,7-dibromo-1,2,3,4-tetrahydroisoquinoline of the present embodiment is as follows:
[0020] (1) Synthesis of N-acetyl 1,2,3,4-tetrahydroisoquinoline
[0021] Take a 100ml single-necked bottle and put it in an ice bath, add 2.66g (0.02mol) of 1,2,3,4-tetrahydroisoquinoline, and add 100mL of dichloromethane, stir to dissolve, and then add 3.03g of triethylamine (0.03mol), after stirring evenly, weigh 2.04g (0.026mol) of acetyl chloride and slowly add it dropwise to the reaction system. Add an appropriate amount of water and dichloromethane to extract 3 times, wash the dichloromethane layer with water 1-2 times, and extract the same washed water layer with dichloromethane 1 time, combine the organic layers, dry the extract with anhydrous sodium sulfate, The solvent was removed by a rotary evaporator, and the residue was purified by column chromatography, eluting with petroleum ether / ethyl acetate=1 / 1 to obtain 3.36 g of a light yellow oil, with ...
Embodiment 2
[0040] The preparation method of 6,7-dibromo-1,2,3,4-tetrahydroisoquinoline of the present embodiment is as follows:
[0041] (1) Synthesis of N-acetyl 1,2,3,4-tetrahydroisoquinoline
[0042] Take a 100ml single-necked bottle, add 2.66g (0.02mol) of 1,2,3,4-tetrahydroisoquinoline, and add 100mL of dichloromethane, stir to dissolve, and then add 2.82g (0.028mol) of triethylamine , after stirring evenly, weigh 1.88g (0.024mol) of acetyl chloride and slowly drop it into the reaction system. After the dropwise addition, the reaction was carried out at room temperature for 3 hours. Afterwards, the reaction solution was poured into a separatory funnel, and an appropriate amount of water and dichloromethane were added thereto for extraction three times. The dichloromethane layer was washed with water once, and the washed water layer was extracted with dichloromethane once again, the organic layers were combined, the extract was dried over anhydrous sodium sulfate, the solvent was r...
Embodiment 3
[0048] The preparation method of 6,7-dibromo-1,2,3,4-tetrahydroisoquinoline of the present embodiment is as follows:
[0049] (1) Synthesis of N-acetyl 1,2,3,4-tetrahydroisoquinoline
[0050] Take a 100ml single-necked bottle, add 2.66g (0.02mol) of 1,2,3,4-tetrahydroisoquinoline, and add 100mL of dichloromethane, stir to dissolve, and then add 2.42g (0.024mol) of triethylamine , after stirring evenly, weigh 1.73g (0.022mol) of acetyl chloride and slowly drop it into the reaction system. After the dropwise addition, the reaction was carried out at room temperature for 3 hours. Afterwards, the reaction solution was poured into a separatory funnel, and an appropriate amount of water and dichloromethane were added thereto for extraction three times. The dichloromethane layer was washed with water once, and the washed water layer was extracted with dichloromethane once again, the organic layers were combined, the extract was dried over anhydrous sodium sulfate, the solvent was r...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com