P-5m-Fc fusion protein and expression gene thereof, and preparation method and applications of P-5m-Fc fusion protein
A technology for fusion protein and gene expression, applied in the field of medicine and biology, can solve the problems of short half-life and poor stability, and achieve the effects of prolonging half-life, improving drugability and improving drug efficacy
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
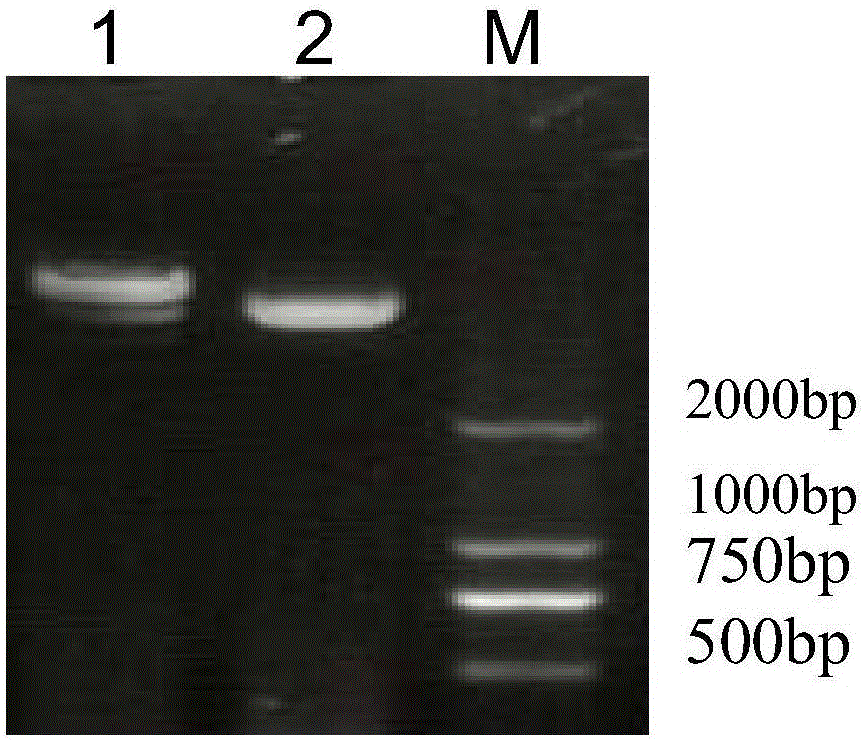
[0057] Construction of expression vector of P-5m-Fc fusion protein
[0058] 1. Method.
[0059] 1. Extraction and purification of bacterial plasmids
[0060] After analysis, the amino acid sequence of P-5m is GHGKHKNK. After screening through a large number of experiments, the inventor synthesized the positive and negative chains of the P-5m peptide gene by using the codons preferred by Escherichia coli, adding ATG to the 5' end and 6 to the 3' end. Glycine codons (GGT GGT GGT GGT GGT GGT part below), and in order to increase the titer, the P-5m peptide gene and glycine codons were repeated for two cycles, after annealing, the 5' end and the 3' end were respectively Generate Nde I recognition site (CAT ATG) and BamH I recognition site (GGA TCC): positive strand 5'-C CAT ATG GGC GAT GGC AAA GAT AAG AAC AAG GGT GGT GGT GGTGGT GGT GGC GAT GGC AAA GAT AAG AAC AAG GGA TCC GCG-3' (the nucleotide sequence shown in SEQ ID NO.2); 5'-CGC GGA TCC CTT GTT CTT ATC TTT GCC ATC GCC CAT ACC...
Embodiment 2
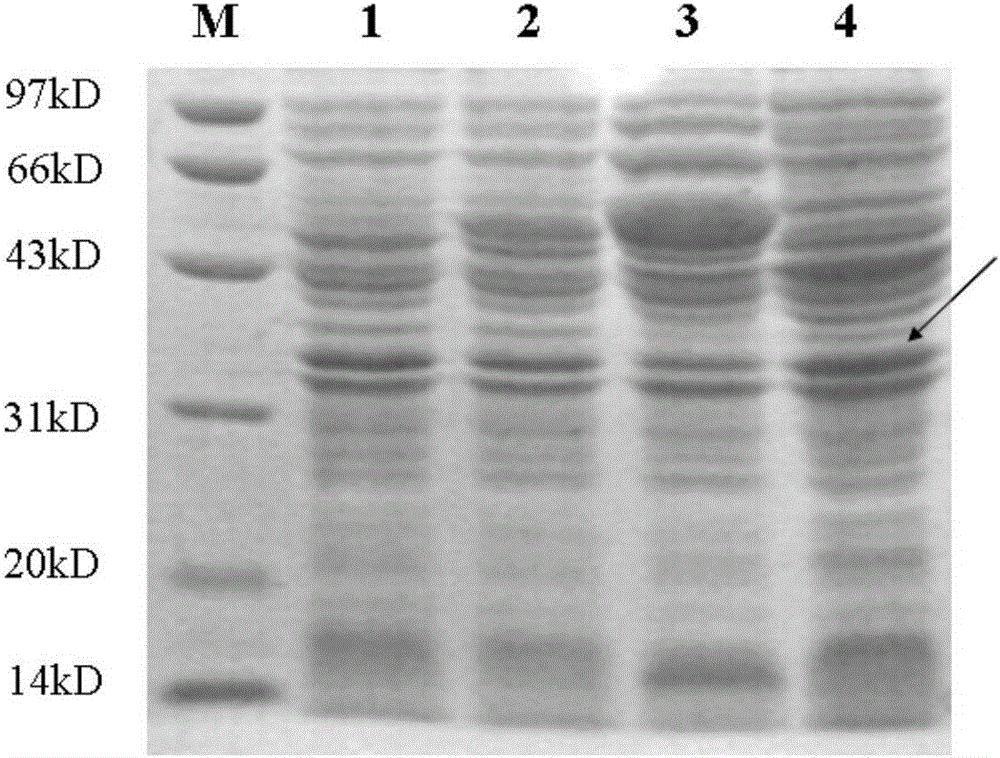
[0085] Expression and purification of recombinant protein in host bacteria
[0086] 1. Method.
[0087] 1. Conversion
[0088] The positive recombinant plasmid p was transformed into E. coli competent BL21 (DE3) by heat shock method, and cultured on LB resistance (containing 50 μg / ml ampicillin) agar plate at 37°C for 12-16h. Pick the recombinant plasmid transformed bacteria of a single strain, inoculate 10ml of LBG medium containing 50μg / ml ampicillin, and culture at 37°C until OD 600 When the concentration reaches 0.4-0.6, add IPTG to a final concentration of 0.3mmol / L, and continue culturing at 200rpm for 3.5h.
[0089] 2. Induced expression
[0090] Take 1ml of the above-mentioned induced product, centrifuge at 5000rpm to induce bacterial culture, discard the supernatant, add 100μL PBS to the precipitate and mix well, add 100μL of 2×SDS loading buffer and mix well, boil for 5min, centrifuge at 8000rpm for 5min at 4°C, take 20μL of supernatant Perform SDS-PAGE electroph...
Embodiment 3
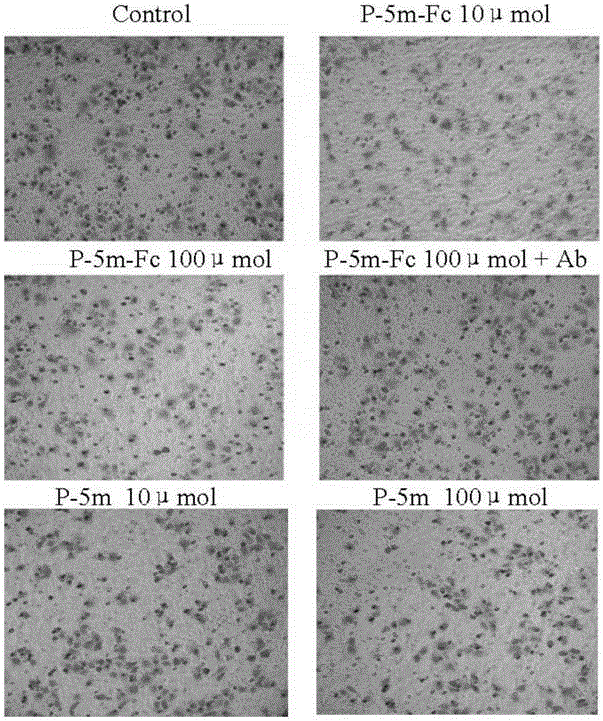
[0123] Activity detection of P-5m–Fc fusion protein
[0124] 1. Western-blotting test
[0125] The protein bands (Fc and P-5m-Fc fusion protein) analyzed by SDS-PAGE were transferred to PVDF membrane, blocked with 1% BSA overnight at 4°C, washed 3 times with 0.05% PBST, 10 min each , join 2000 × Diluted positive serum, acted at 37°C for 2 hours, washed 3 times with 0.05% PBST, 10 minutes each time, added 25000 × Diluted rabbit anti-human IgG enzyme-labeled antibody, washed 3 times with 0.05% PBST, 10 minutes each time, added PIERCE luminescent substrate to react at room temperature for 3 minutes, exposed in the dark room, fixed after developing, the result is as follows Image 6 shown.
[0126] The above results indicated that Fc could not bind to P-5m polyclonal antibody, but P-5m-Fc could specifically bind to P-5m polyclonal antibody.
[0127] 2. Indirect ELISA test
[0128] 1. Preparation of polyclonal antibody against P-5m octapeptide
[0129] (1) Antigen preparation...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com