Medical hemostatic sponge and preparation method thereof
A hemostatic sponge and sponge technology are applied in the field of medical hemostatic sponge and its preparation, which can solve the problems of poor crystal form of chitosan sponge, cumbersome post-processing steps, long sponge degradation cycle, etc. Handling the effect of simple, fast and convenient promotion
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
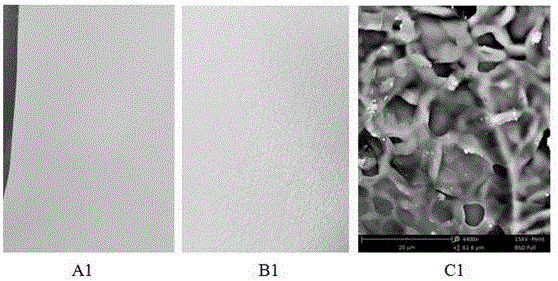
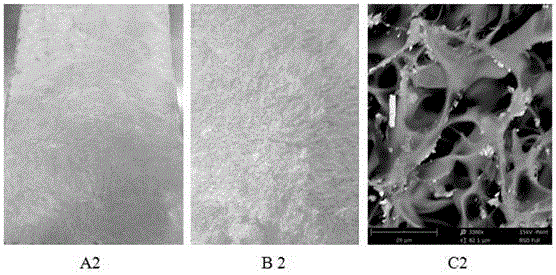
Image
Examples
Embodiment 1
[0064] Adopt the following method to prepare medical hemostatic sponge:
[0065] 1. Weigh 100g of chitosan, add 500mL of ethanol, slowly add 40g of 50% acetic acid solution dropwise, stir for two hours until the salt is completely formed, filter and wash the excess acid, and dry to obtain chitosan salt.
[0066] 2. Weigh 100g of chitosan salt, add 4.5L of water for injection, then add 0.5L of tert-butanol and stir to dissolve, filter the dissolved solution, and the filtrate will enter the next step for future use.
[0067] 3. Divide the filtrate into freeze-drying molds with a thickness of 2mm. Put the divided molds into a vacuum freeze dryer, slowly cool down to -35°C, keep for 1 hour, then slowly raise the temperature to 0°C, and keep at 0°C ℃ until the product temperature reaches above 0 ℃ for 1 hour, then warm up to room temperature and continue to dry for 2 hours and shut down to obtain chitosan sponge a.
[0068] 4. Weigh 100g carboxymethyl chitosan, add 4.5L water for ...
Embodiment 2
[0072] Operation process is identical with embodiment 1, only replaces the acetic acid in embodiment 1 with hydrochloric acid.
Embodiment 3
[0074] Operation process is identical with embodiment 1, only replaces the acetic acid in embodiment 1 with lactic acid.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Thickness | aaaaa | aaaaa |
| Density | aaaaa | aaaaa |
| Thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com