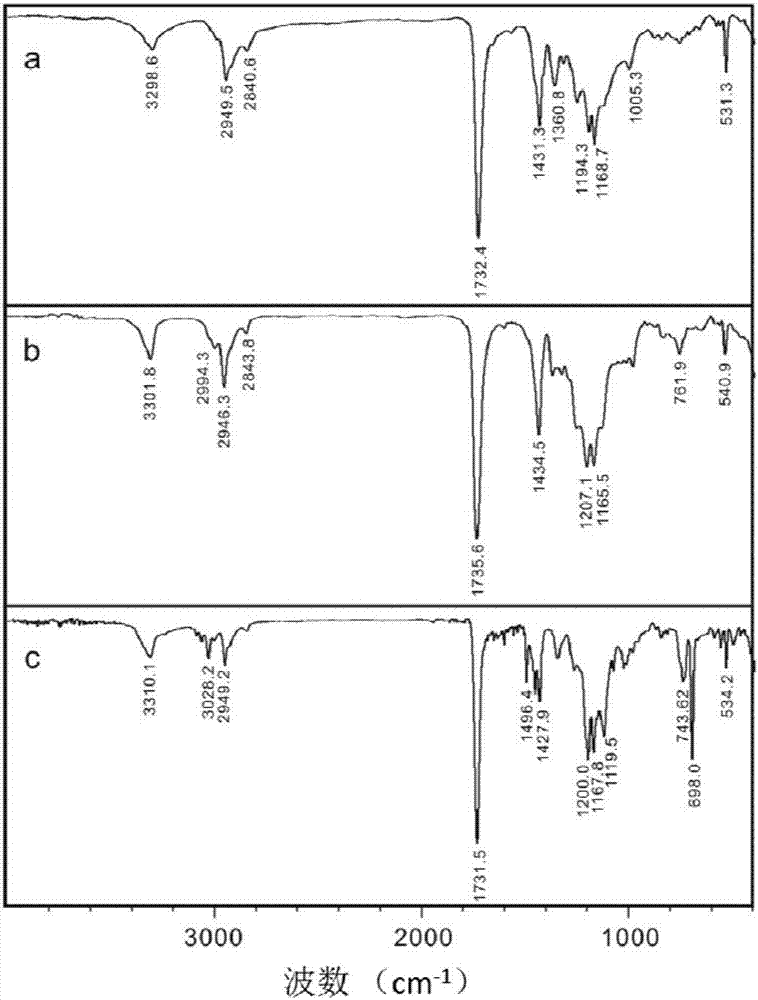
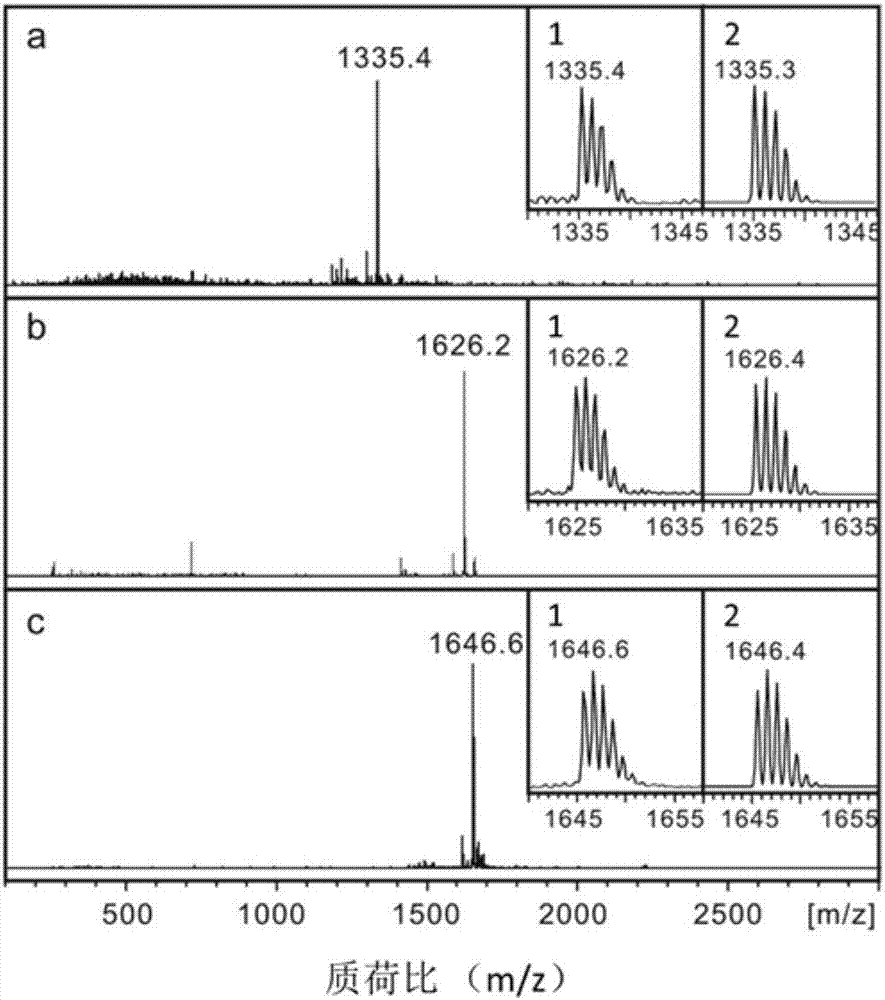
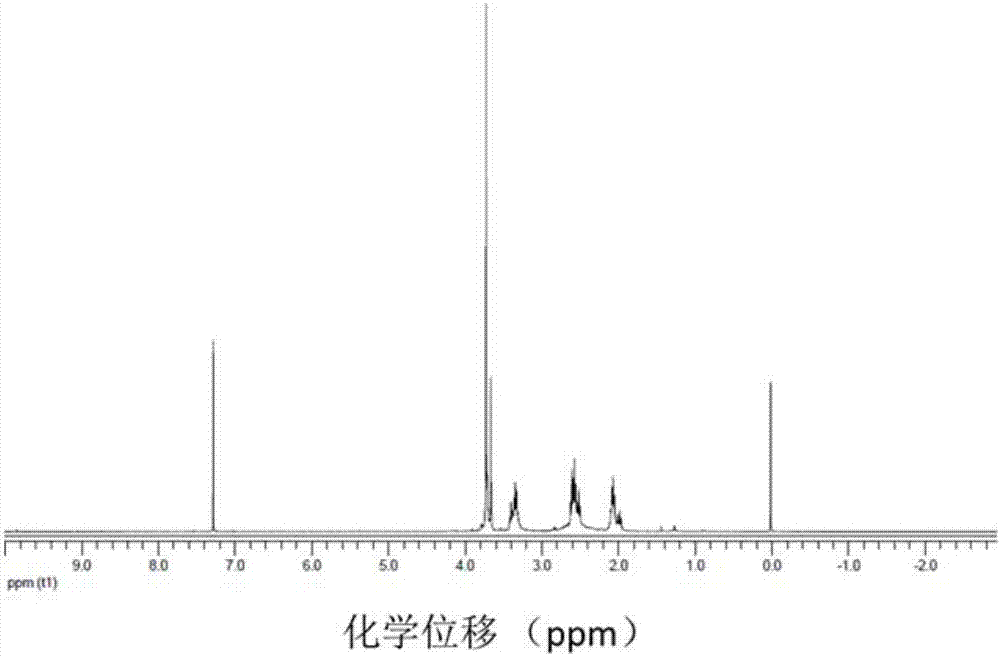
A class of fullerene amino acid ester self-assembled drug-loaded sustained-release vesicle material and its preparation method and application
A technology of fullerene amino acid esters and amino acid esters, which is applied in the field of fullerenes, can solve problems such as hindering biological activity and application in the medical field, hindering assembly research, etc., to solve hydrophobic properties, simple preparation methods, and satisfactory preparation conditions Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0044] 1) 500mg of fullerene C 60 Dissolve in 150mL chlorobenzene, stir magnetically at room temperature for 4h to dissolve it completely, add 3.5g ICl, react at room temperature for 4h, use oil pump to remove excess ICl and chlorobenzene solvent, and obtain an orange-yellow solid. Wash 3 times with n-hexane, let it stand, remove the supernatant, keep the solid, and dry to obtain the product;
[0045] 2) HCl gas will be passed into 250mL of methanol to form a saturated methanolic hydrochloric acid solution, and 10.31g of 4-aminobutyric acid methyl ester will be added. At this time, 4-aminobutyric acid methyl ester will form 4-aminobutyric acid methyl ester hydrochloride Dissolve in methanol, heat to 65°C and reflux for 20 minutes, use a rotary evaporator to spin dry the product to remove solvent and excess HCl, and after drying, obtain methyl 4-aminobutyrate dimethyl hydrochloride;
[0046] 3) Add 3.83g of 4-aminobutyric acid methyl ester hydrochloride into 250mL of ethyl ace...
Embodiment 2
[0052] 1) 500mg of fullerene C 60 Dissolve in 150mL chlorobenzene, stir magnetically at room temperature for 4h to dissolve it completely, add 3.5g ICl, react at room temperature for 4h, use oil pump to remove excess ICl and chlorobenzene solvent, and obtain an orange-yellow solid. Wash 3 times with n-hexane, let it stand, remove the supernatant, keep the solid, and dry to obtain the product;
[0053] 2) HCl gas will be passed into 250mL of methanol to form a saturated methanolic hydrochloric acid solution, and 10.31g of 4-aminobutyric acid methyl ester will be added. At this time, 4-aminobutyric acid methyl ester will form 4-aminobutyric acid methyl ester hydrochloride Dissolve in methanol, heat to 65°C and reflux for 20 minutes, use a rotary evaporator to spin dry the product to remove solvent and excess HCl, and after drying, obtain methyl 4-aminobutyrate dimethyl hydrochloride;
[0054] 3) Add 3.83g of 4-aminobutyric acid methyl ester hydrochloride into 250mL of ethyl ace...
Embodiment 3
[0059] 1) 500mg of fullerene C 60 Dissolve in 150mL chlorobenzene, stir magnetically at room temperature for 4h to dissolve it completely, add 3.5g ICl, react at room temperature for 4h, use oil pump to remove excess ICl and chlorobenzene solvent, and obtain an orange-yellow solid. Wash 3 times with n-hexane, let it stand, remove the supernatant, keep the solid, and dry to obtain the product;
[0060] 2) HCl gas will be passed into 250mL of methanol to form a saturated methanolic hydrochloric acid solution, and 10.31g of 4-aminobutyric acid methyl ester will be added. At this time, 4-aminobutyric acid methyl ester will form 4-aminobutyric acid methyl ester hydrochloride Dissolve in methanol, heat to 65°C and reflux for 20 minutes, use a rotary evaporator to spin dry the product to remove solvent and excess HCl, and after drying, obtain methyl 4-aminobutyrate dimethyl hydrochloride;
[0061] 3) Add 3.83g of 4-aminobutyric acid methyl ester hydrochloride into 250mL of ethyl ace...
PUM
| Property | Measurement | Unit |
|---|---|---|
| radius | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com