Pharmaceutical composition of chlorprothixene and protective effect on cerebral ischemia reperfusion injury
A technology of cerebral ischemia-reperfusion and chlorprothixol, which is applied in the field of biomedicine, can solve the problems that have not yet been reported on the correlation of chlorprothixol cerebral ischemia-reperfusion injury, and achieve the reduction of cerebral ischemia-reperfusion injury, Excellent preventive effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
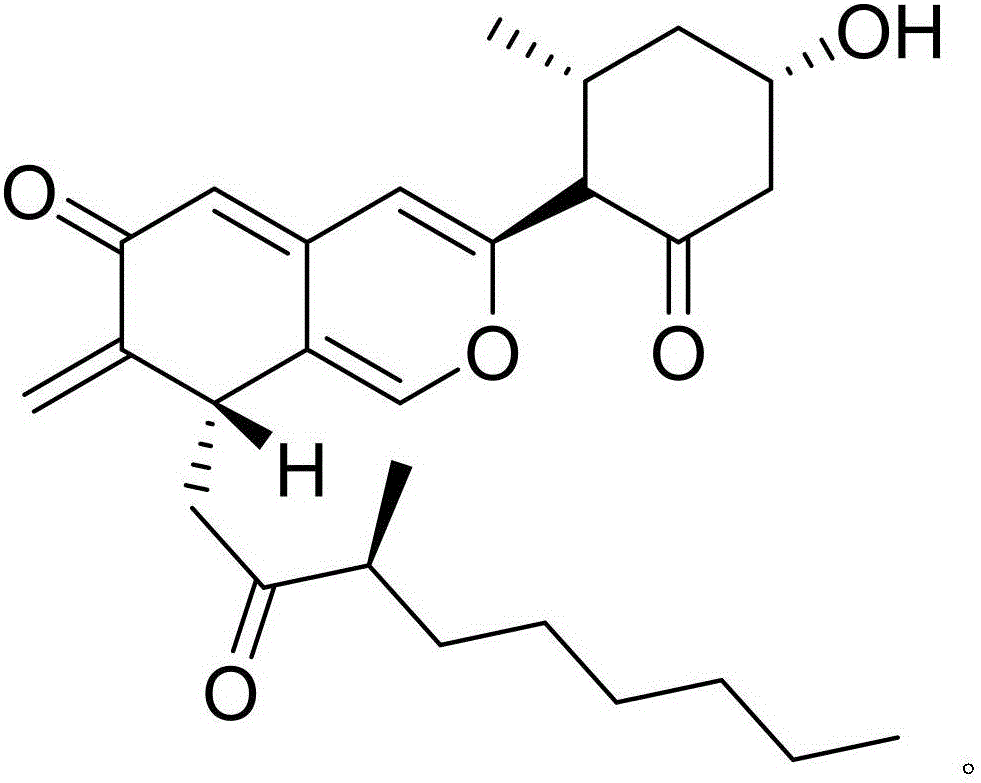
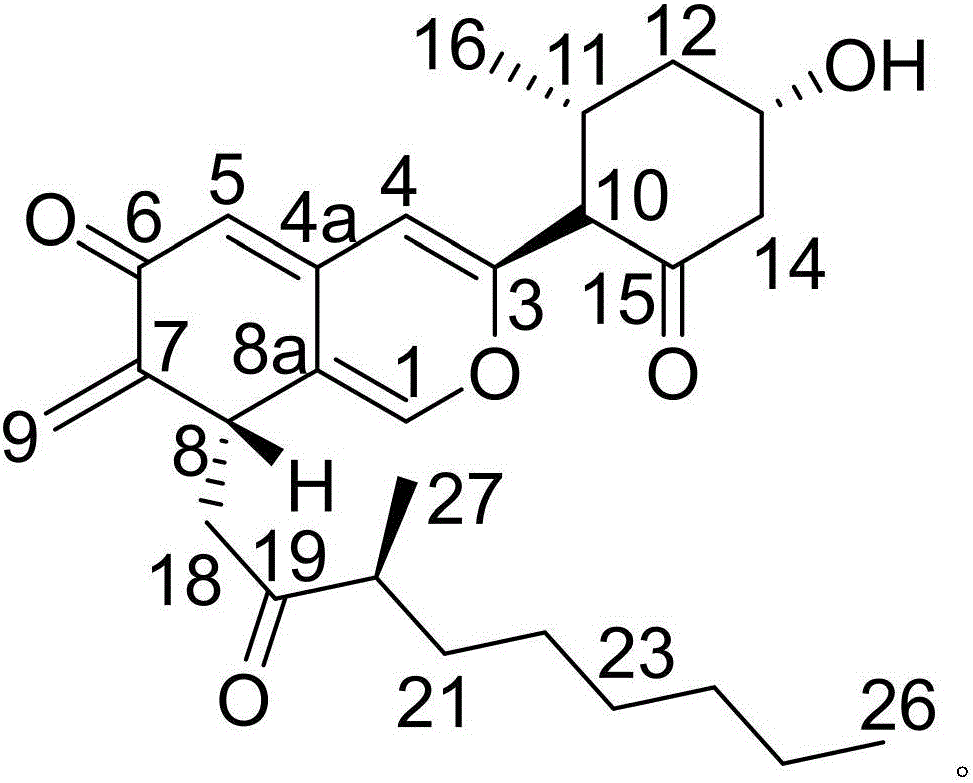
[0017] Example 1: Compound (I) Separation Preparation and Structure Confirmation
[0018] Separation method: (a) Pulverize Nansha ginseng (2kg), extract with 65% ethanol under hot reflux (20L×3 times), combine the extracts, concentrate until there is no alcohol smell (4L), and successively use petroleum ether (4L×3 times) ), ethyl acetate (4L × 3 times) and water-saturated n-butanol (4L × 3 times) extraction, respectively obtain petroleum ether extract, ethyl acetate extract and n-butanol extract; (b) step (a ) in n-butanol extract with AB-8 type macroporous resin for impurity removal, first elute 12 column volumes with 10% ethanol, then elute 15 column volumes with 70% ethanol, collect 70% eluate, decompress Concentrate to get 70% ethanol elution concentrate; (c) in step (b), 70% ethanol elution concentrate is separated with normal phase silica gel, and the volume ratio is 50:1 (8 column volumes), 25:1 ( 8 column volumes), 15:1 (8 column volumes) and 5:1 (10 column volumes) ...
Embodiment 2
[0021] Example 2: Prevention and treatment of rat cerebral ischemia-reperfusion injury model
[0022] 1. Materials and methods
[0023] 1.1 Animals
[0024] Sixty adult healthy male Sprague-Dawley (SD) rats, SPF grade, weighing 280-300 g, were provided by Beijing Weitong Lihua Experimental Animal Technology Co., Ltd. During the experiment, the feeding environment of the rats was stable, with room temperature (25±2)°C, relative humidity (55±5)%, and light control for 12 hours to alternate day and night.
[0025] 1.2 Reagents and samples
[0026] Chlorprothixene was purchased from Shanghai Jiwei Biotechnology Co., Ltd. Compound (I) is self-made, and the preparation method is shown in Example 1. Edaravone injection (Sinopharm Guorui Pharmaceutical Co., Ltd.). Rabbit anti-mouse GFAP antibody, rabbit anti-mouse Iba1 antibody were purchased from Vector Company; rabbit anti-mouse TNF-α antibody, rabbit anti-mouse IL-1β, rabbit anti-mouse VCAM-1 antibody, rabbit anti-mouse ICAM-1...
PUM
| Property | Measurement | Unit |
|---|---|---|
| degree of unsaturation | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com