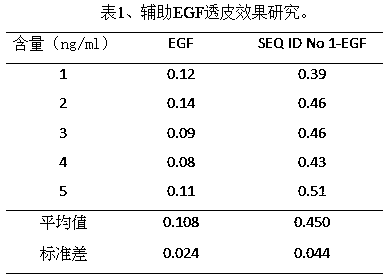
A kind of transdermal peptide and its application
A transdermal and skin technology, applied in the field of bioengineering, can solve the problems of pain, poor flexibility of dosage forms, high cost, and achieve the effect of transdermal absorption
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0015] Example 1. Polypeptide SEQ ID No 1: ACSSTKKHCG was synthesized by solid phase synthesis.
[0016] Microwave-assisted solid-phase synthesis of peptides was adopted: Wang resin was used as a solid-phase carrier, HBTU-HOBt was used as a condensation agent, and Fmoc was used as a synthetic strategy for the α-amino protecting group. Weigh 0.1 mmol of Fmoc-glycine-Wang resin into a solid-phase reactor, add 10 mL of DCM to swell the resin for 30 min, wait until the resin is fully swollen, and vacuum the solvent to dry up. Wash once with 20% piperidine / DMF10mL, add 20% piperidine / DMF10mL to the resin and place it in a microwave-assisted solid-phase synthesizer, react at 60°C and 20W for 3min to remove the Fmoc protecting group, then use DCM, Washing with DMF, removal of protective group is detected by Kaiser method, if the detection is positive, the reaction can be continued. Dissolve 3eq of Fmoc-amino acid-OH, HBTU and HOBt in DMF, add dropwise 5eq of DIEA and let it stand fo...
Embodiment 2
[0017] Embodiment 2, animal experiments verify transdermal activity
[0018] This example is to study the transdermal effect of the transdermal polypeptide at the mouse level, and the specific steps are as follows.
[0019] 1. Shave the mice 36 hours before the experiment to obtain Hairless skin.
[0020] 2. Bacteria solution (purchased from Beijing Tianenze) was added to 4 mL medium, incubated at 37°C, and centrifuged at 180 rpm for 4h.
[0021] 3. The mice were anesthetized with 3.5% chloral hydrate, the anesthesia dose was .
[0022] 4. After each mouse is prepared skin surface, plus Phage.
[0023] 5. Take blood for half an hour and one hour respectively , mixed and added to 1 mL of the bacterial solution prepared in step 2, incubated at 37°C, and centrifuged at 120 rpm for 40 minutes.
[0024] 6. Use the blue-white screening method for the second-step mixture overnight to obtain figure 1 The results shown.
[0025] 7. Pick blue spots, amplify and extrac...
Embodiment 3
[0026] Example 3. Research on Transdermal Peptide Assisted siRNA Transdermal Activity
[0027] This example is to study the transdermal effect of the transdermal polypeptide at the level of rats, and the specific steps are as follows.
[0028] 1. Shave the rats 36 hours before the experiment to get Hairless skin.
[0029] 2. Thoroughly mix a small amount (about 0.1 g) of cosmetic base with 1 mg of chemically synthesized SEQ ID No1 polypeptide and fluorescently labeled siRNA (100 ng).
[0030] 3. Spread the mixture evenly on the skin of rats that have been depilated, and wrap the skin with plastic wrap.
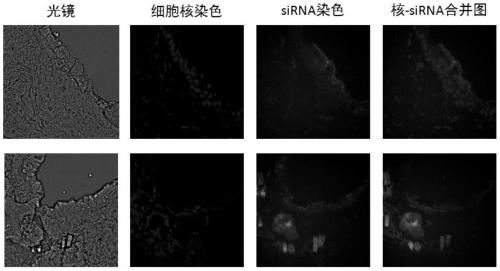
[0031]4. After 1 hour, the rats were sacrificed, the skin was separated, and the skin surface was washed with PBS, and then the skin samples were embedded and frozen into sections under liquid nitrogen quick-freezing conditions. The transdermal effect of siRNA was observed by confocal microscopy. get figure 2 The results shown. From the figure, we can see that the skin...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com