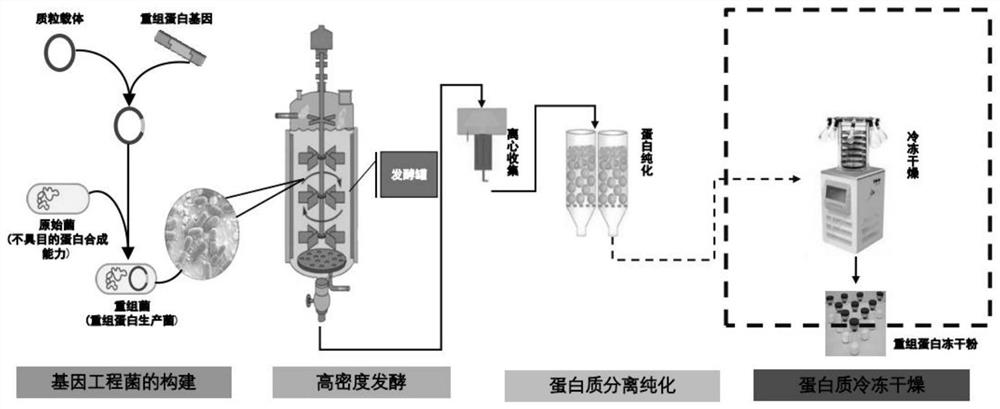
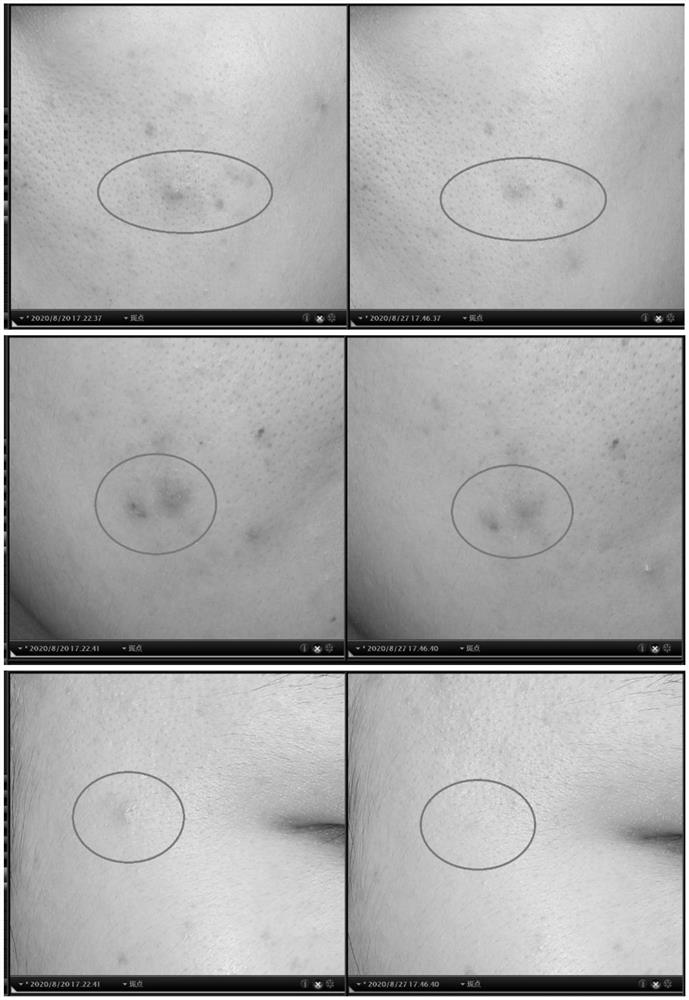
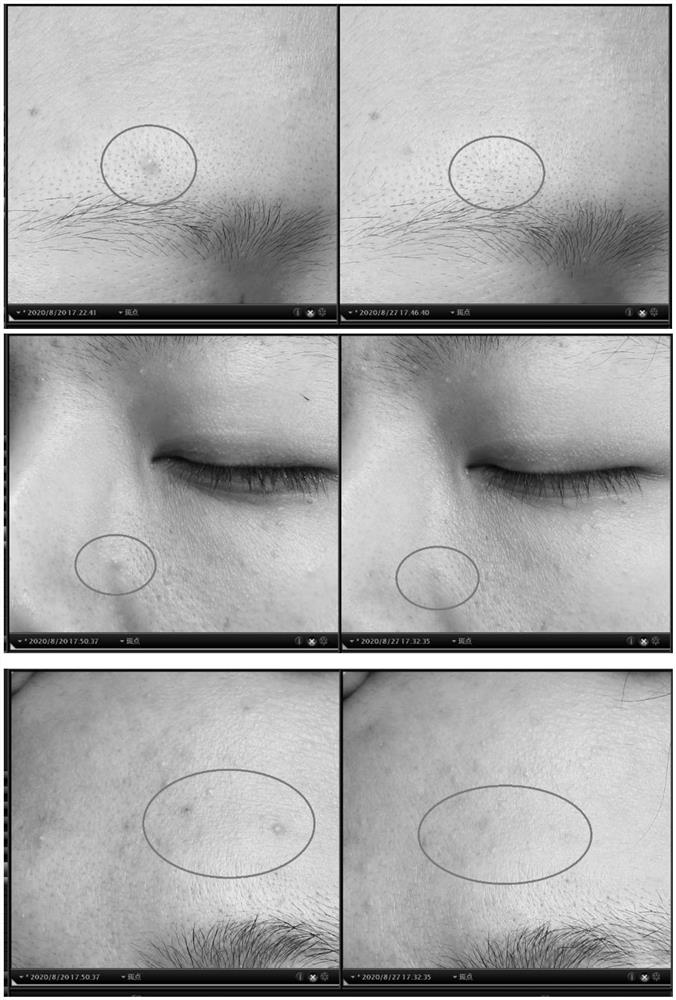
Active protein peptide gene with excellent repairing effect and encoded protein peptide
A technology of protein peptides and vitality, applied in the field of active peptides, can solve the problems of high cost, difficulty in fully exerting efficacy, difficulty in mass production of EGF, etc., and achieve the effect of high safety
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] Example 1: Obtaining the target DNA
[0028] (1) The gene sequence of the target gene is:
[0029] Using the messenger RNA transcribed by 5'-GCAAGCGCATTGCACATGTACGGCTATAGCAGATA-3' (SEQ ID NO: 1) as a template, it is reverse-transcribed into complementary single-stranded DNA, and then synthesized into double-stranded DNA under the action of T4 DNA ligase, which is the target gene.
[0030] (2) PCR amplification
[0031] Denaturation: High temperature dissociates double-stranded DNA into single strands (94°C, 30s);
[0032] Annealing: The primer (pACT2 GAL4 AD pACT2-R) binds to the complementary region of the template DNA at low temperature to form a hybrid molecule (55°C, 30s);
[0033] Extension: moderate temperature extension, in DNA polymerase, dNTP, Mg 2+ In the presence, DNA polymerase catalyzes the extension of the DNA strand starting from the primer from 5' to 3', and synthesizes a DNA sub-strand complementary to the template DNA strand (70-72°C, 30-60s);
[0...
Embodiment 2
[0035] Embodiment 2: Electrophoresis detection and recovery purification
[0036] (1) Electrophoresis
[0037] ① Prepare an appropriate amount of buffer for electrophoresis and gel preparation (1X TAE Buffer)
[0038] ② Accurately weigh the agarose powder (1g) according to the amount of gel to be prepared and the concentration of the gel, and add it to an appropriate Erlenmeyer flask.
[0039] ③ Add a certain amount of electrophoresis buffer (1X TAE Buffer 100ml).
[0040] ④ Melt completely, cool to about 60°C, add EB5-7ul, and mix well.
[0041] ⑤ Pour the solution into the rubber mold, before inserting the comb in the appropriate position.
[0042] ⑥It solidifies at room temperature. If it is not used immediately, wrap the gel with plastic wrap and store it at 4°C.
[0043] Mix an appropriate amount of PCR product with an appropriate amount of bromophenol blue and add it to the gel tank, and add an appropriate amount of DNAMaker to the right tank to start electrophoresis...
Embodiment 3
[0049] Embodiment 3: Recombinant expression vector construction
[0050] The selected recombinant expression vector plasmid vector is pBR322, and the construction of the recombinant plasmid includes the following steps:
[0051] (1) Since one of the parents of the pBR322 plasmid is the pMB1 plasmid, at first based on pMB1, the Tn3 translocation of the Rldrd19 plasmid is introduced to obtain the pMB3 plasmid of 13.3kb;
[0052](2) The molecular weight of pMB3 obviously makes it unsuitable as a carrier, so most of the useless fragments must be lost by digestion under EcoRI active conditions, and the sticky ends of the remaining small fragments of DNA are connected to form pMB8 plasmid (2.6kb);
[0053] (3) At this time, another pSC101 plasmid was digested under EcoRI activity conditions to produce a DNA fragment containing tetr resistance, and this fragment was integrated with pMB8 to form pMB9 plasmid (5.3kb). At this time, pMB9 is ampstetr phenotype;
[0054] (4) pMB9 has p...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com