Iron oxide red pigment and preparation method thereof
A technology of iron oxide red and pigment, which is applied in the field of preparation of low heavy metal full liquid phase method iron oxide red by titanium dioxide by-product ferrous sulfate, and achieves the effect of fine particles, saving iron resources and improving automation.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
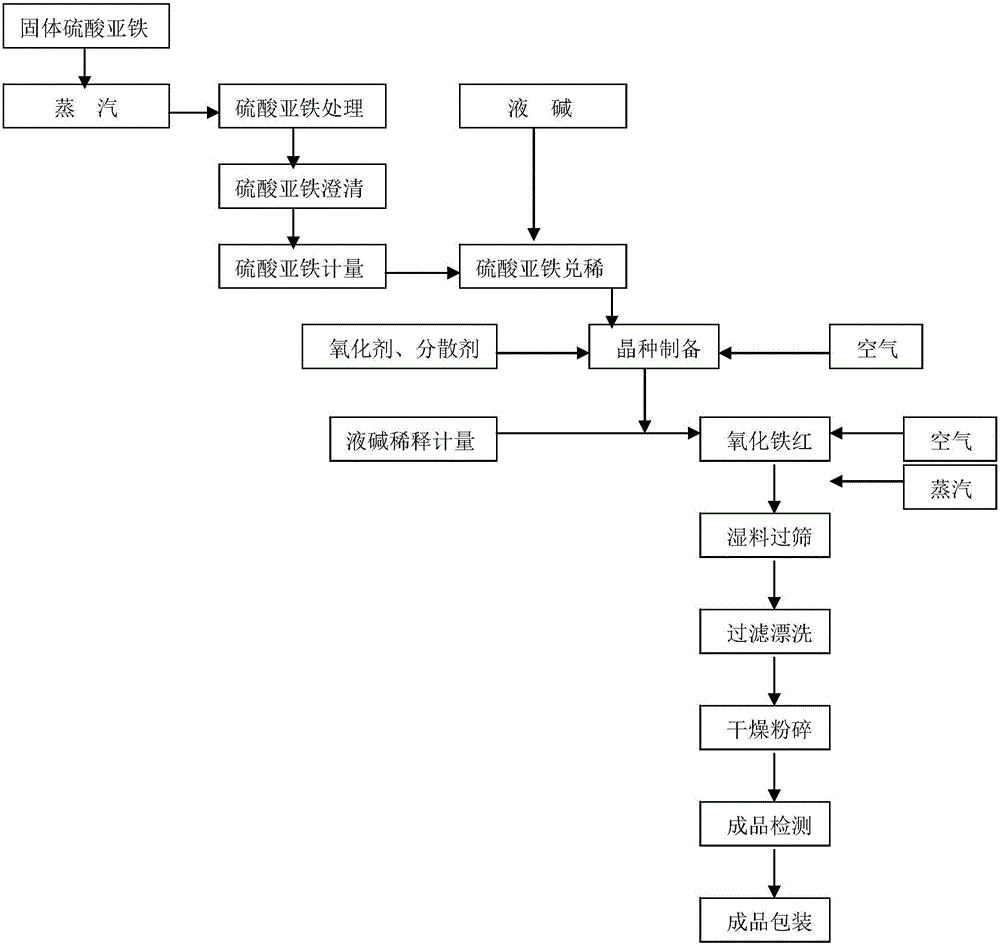
[0026] Get 300ml of refined ferrous sulfate, dilute it with water to a concentration of 1.0g / L, put it into a reactor equipped with an air device, add 0.02% dispersant propylene glycol and hydrogen peroxide, and quickly (complete within five minutes) add Use 32% liquid caustic soda to make the pH reach 9, and ventilate the air to maximize the air. The oxidation reaction takes 15 minutes until the color is brownish red, and iron red seed crystal solution is obtained.
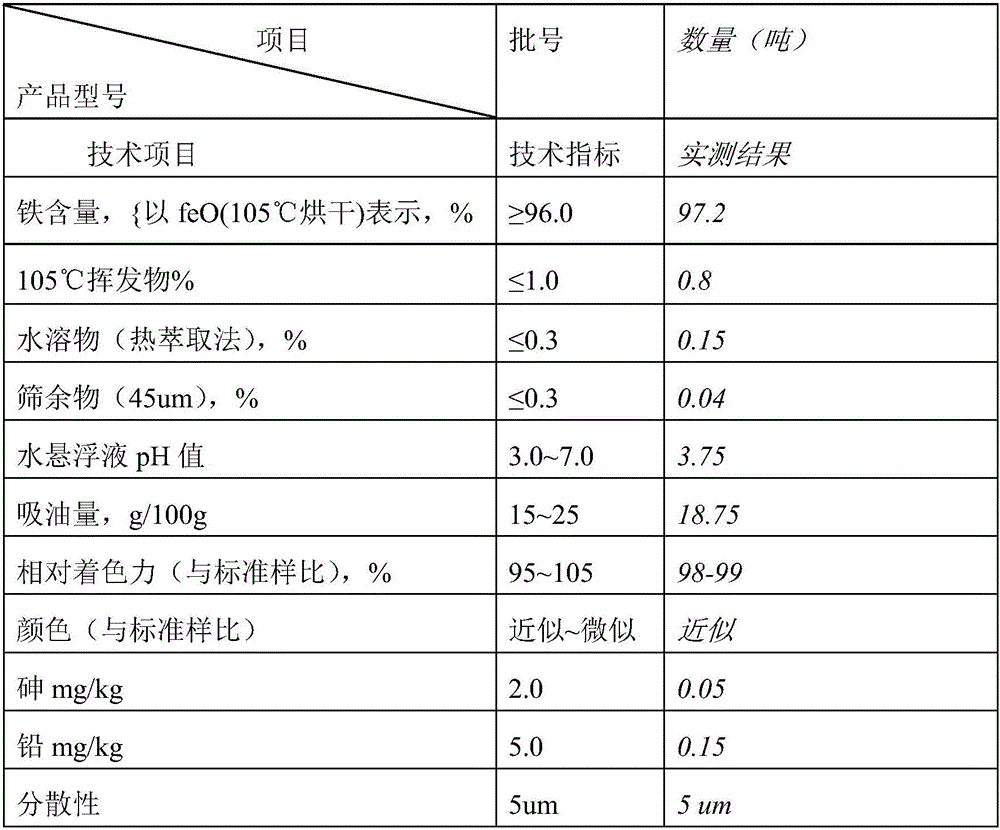
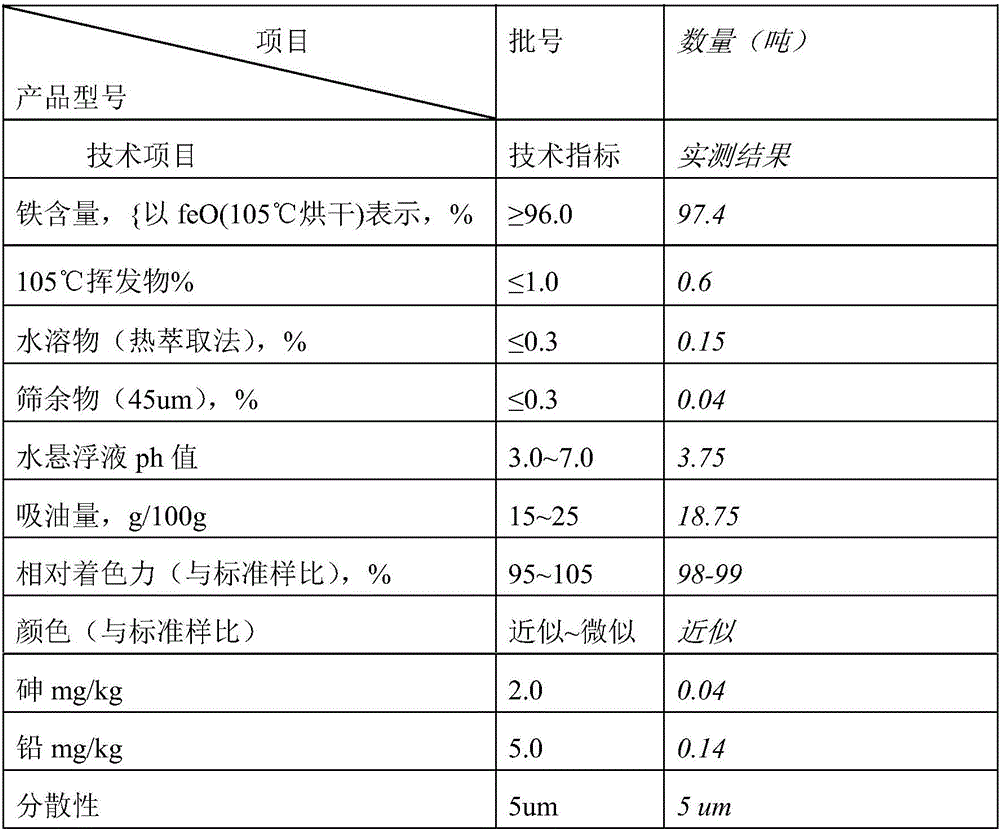
[0027] Within 5 minutes, take the above-mentioned seed crystals and add them to the oxidation tank, adjust the pH value to about 5 with dilute sulfuric acid, add refined ferrous sulfate, heat up to 60°C with steam, and add about 10% concentrated alkali dropwise at the same time. Control the pH value between 2.5-3.0 until the material liquid hair gel turns into a yellow-red phase; at this time, raise the temperature to 80°C, continue to control the pH value between 3.5-4.5, oxidize for 16 hours, and reach the ideal...
Embodiment 2
[0031] Get refined ferrous sulfate 300ml. Dilute to a concentration of 0.4g / L and put it into a reactor equipped with air, add 0.02% dispersant propylene glycol and hydrogen peroxide, quickly add 32% liquid caustic soda to pH 10, and ventilate to maximize the air to oxidize the seed crystal quickly, and the oxidation reaction lasted for 20 minutes until the color was brownish red, and the iron red seed crystal solution was obtained.
[0032] Take the above-mentioned seed crystals and add them to the oxidation tank within 5 minutes, adjust the pH to about 6 with dilute sulfuric acid, add a certain amount of refined ferrous sulfate, raise the temperature to 60°C, and add about 10% concentrated alkali dropwise to control the pH at the same time. Between 2.5-3.0, until the material liquid hair gel turns into a yellow-red phase; at this time, raise the temperature to 85°C, continue to control the pH between 3.5-4.5, oxidize for 16-30 hours, and reach the ideal color, put it in a bu...
Embodiment 3
[0036] Get 300ml of refined ferrous sulfate, dilute to a concentration of 0.5g / L and put it into a reactor equipped with air loading, add 0.02% dispersant propylene glycol and hydrogen peroxide, add quickly with 32% liquid caustic soda to make the pH reach 11, The air was ventilated to maximize the air, and the reaction was carried out for 25 minutes until the color was brownish red to obtain an iron red seed crystal solution.
[0037] Take the above-mentioned seed crystals and add them to the oxidation tank within 5 minutes, adjust the pH to about 7 with dilute sulfuric acid, add a certain amount of refined ferrous sulfate, raise the temperature to 60°C, and add about 10% concentrated alkali dropwise to keep the concentration of the material. The pH is between 2.5-3.0, until the material liquid hair gel turns into a yellow-red phase; at this time, the temperature is raised to 80°C, and the pH is continued to be controlled between 3.5-4.5, and the oxidation reaction takes 16-30...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com