Morusin skeleton splicing convolutamydine A skeleton compound and its preparation method and application
A technology of compounds and skeletons, applied in the field of chemistry, to achieve the effects of easy-to-obtain raw material synthesis, cheap raw material synthesis, and good air stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
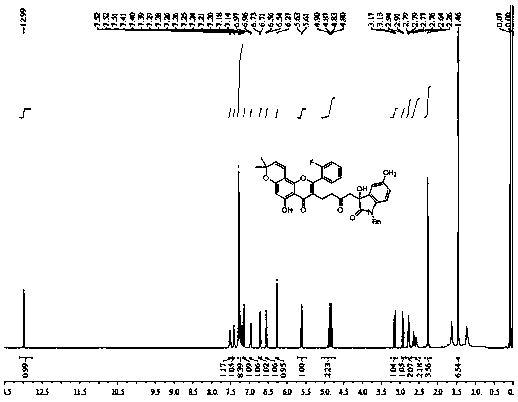
[0020] The preparation method of compound 3b-3w is the same as that of compound 3a, and the feeding ratio is the same as that of compound 3a. Compound 3b-3w can be obtained. The reaction yield is shown in Table 1 and Table 2, but it should be emphasized that the compound of the present invention is not limited to Table 1 and Table 2 2 represents the content.
[0021] Table 1 is the chemical structure of a Morusin skeleton splicing convolutamydine A skeleton compound
[0022]
[0023] Table 2 is the chemical structure of a Morusin skeleton splicing convolutamydine A skeleton compound
[0024]
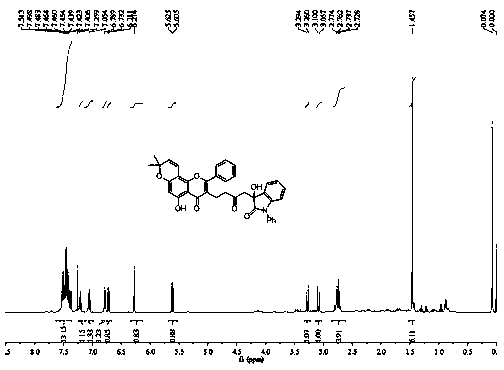
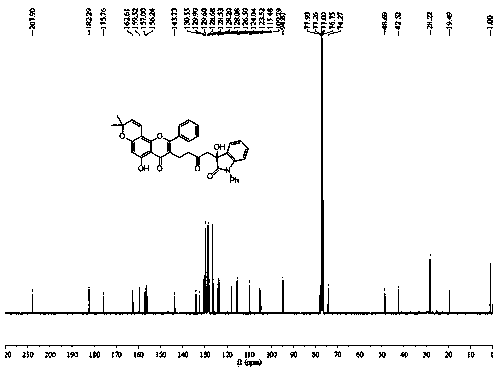
[0025] Compound 3b prepared in this example: yellow solid, melting point: 75.6-76.2 o C, productive rate 62%; Results such as NMR and high-resolution mass spectrometry test are as follows: 1 H NMR (CDCl 3 , 400 MHz) δ : 1.46 (s, 6H), 2.26 (s,3H), 2.62-2.66 (m, 2H), 2.76-2.79 (m, 2H), 2.93 (d, J = 12.1 Hz, 1H), 3.15(d, J = 12.5 Hz, 1H), 4.80-4.90 (m, 2H), 5.62 (d, J = 7.8...
Embodiment 1
[0048] Pharmacological Example 1: Cytotoxicity of Compounds 3b-3w (excluding 3l) to K562 Cells
[0049] K562 (human chronic myeloid leukemia cells) were cultured in DMEM medium containing 10% fetal bovine serum, 100 U / mL penicillin and 100 U / mL streptomycin. Cells were added to 96 wells at a concentration of 4000 cells per well at 37°C with 5% CO 2 Incubate for 24 hours in a humidified incubator.
[0050] Cell viability was determined by the modified MTT method. After the cells were incubated for 24 hours, the newly prepared dimethyl sulfoxide solution of compounds 3b-3w (excluding 3l) was added to each well in a concentration gradient, so that the final concentrations of the compounds in the wells were 3 μmol / L , 6 μmol / L, 13 μmol / L, 25 μmol / L and 50 μmol / L. After 48 hours, 10 μL of MTT (5 mg / mL) in phosphate buffer was added to each well, and then continued at 37 o C After 4 hours of incubation, centrifuge for 5 minutes to remove unconverted MTT, and add 150 μL dimethyl ...
Embodiment 2
[0052] Pharmacological Example 2: Cytotoxicity of Compounds 3b, 3d, 3e, 3j, 3r and 3v on PC-3 cells
[0053] PC-3 (human prostate cancer) cells were cultured in RPMI-1640 medium containing 10% fetal bovine serum, 100U / mL penicillin and 100U / mL streptomycin. Cells were added to 96 wells at a concentration of 5000 cells per well, at 37 o C with 5% CO 2 Incubate for 24 hours in a humidified incubator.
[0054] Cell viability was determined by the modified MTT method. After the cells were incubated for 24 hours, newly prepared dimethyl sulfoxide solutions of compounds 3b, 3d, 3e, 3j, 3r and 3v were added to each well in a concentration gradient, so that the final concentrations of the compounds in the wells were 3 μmol / L, 6 μmol / L, 13 μmol / L, 25 μmol / L and 50 μmol / L. After 48 hours, 10 μL of MTT (5 mg / mL) in phosphate buffer was added to each well, and then continued at 37 o C After 4 hours of incubation, centrifuge for 5 minutes to remove unconverted MTT, and add 150 μL dime...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com