Application of a compound in preparation of medicine for treating Parkinson's disease
A Parkinson's disease, compound technology, applied in the field of pharmaceuticals, can solve problems such as loss of ability to produce dopamine, reduced buffering capacity of exogenous dopamine drugs, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
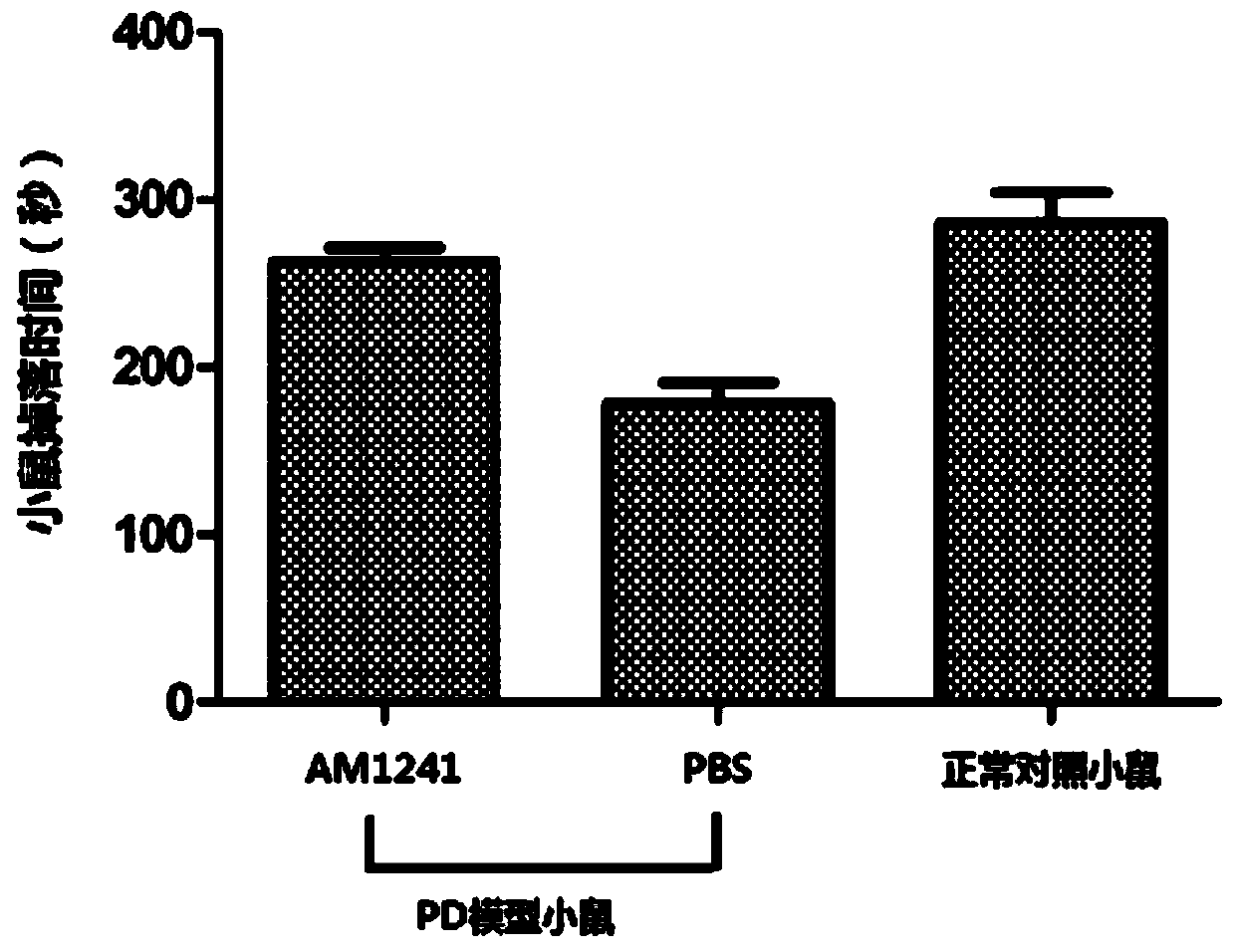
[0031] Example 1 Pharmacodynamic experiment of AM1241 in treating Parkinson's disease in mice
[0032] First, the classical MPTP method was used to establish a subacute model of PD in C57 mice: MPTP (mg) required for each mouse: mouse body weight (0.02g) × 30mg / kg (MPTP) × administration time (5d). After intraperitoneal injection for 5 consecutive days (dosing at a fixed time every day), the mice were left for 5 days to evaporate the remaining MPTP in the cage, and the mouse rotarod experiment analysis showed that the success rate of modeling was as high as 85%.
[0033] In the second step, the successfully modeled PD mice were divided into random groups, 6 in each group, administered with PBS and AM1241 (AM1241 used in this embodiment, purchased from Selleck China), and a normal control group was set at the same time. The dose of AM1241 was (1.5mg / kg / d), administered by intraperitoneal injection, and administered continuously for 2 weeks. During the experiment, the mice were...
Embodiment 2
[0046] The preparation experiment of the solid lipid nanoparticle dosage form of embodiment 2 AM1241
[0047] (1) Synthesis steps of SLN-AM1241:
[0048] (1) Weigh 0.25g surfactant Myrj59, dissolve in 30ml double distilled water to form water phase;
[0049] (2) Fix the three-neck flask in a constant temperature magnetic stirring water bath, and add the prepared water phase when the water temperature rises to 75°C;
[0050] (3) Weigh 0.25g of stearic acid, 0.1g of lecithin, 20mg of AM1241, mix and dissolve in 10ml of chloroform, oscillate and ultrasonically mix, and inject into the water phase at a constant speed with a 6# syringe;
[0051] (4) Stir at 1000rpm for about 0.5h. When the mixed solution is milky, add 10ml of cooled double distilled water, and place the three-neck flask on ice and continue to stir for 2h;
[0052] (5) After stirring, transfer the sample to a centrifuge tube, wash twice with double distilled water at 4°C and 20,000rpm; freeze-dry the washed sample...
Embodiment 3
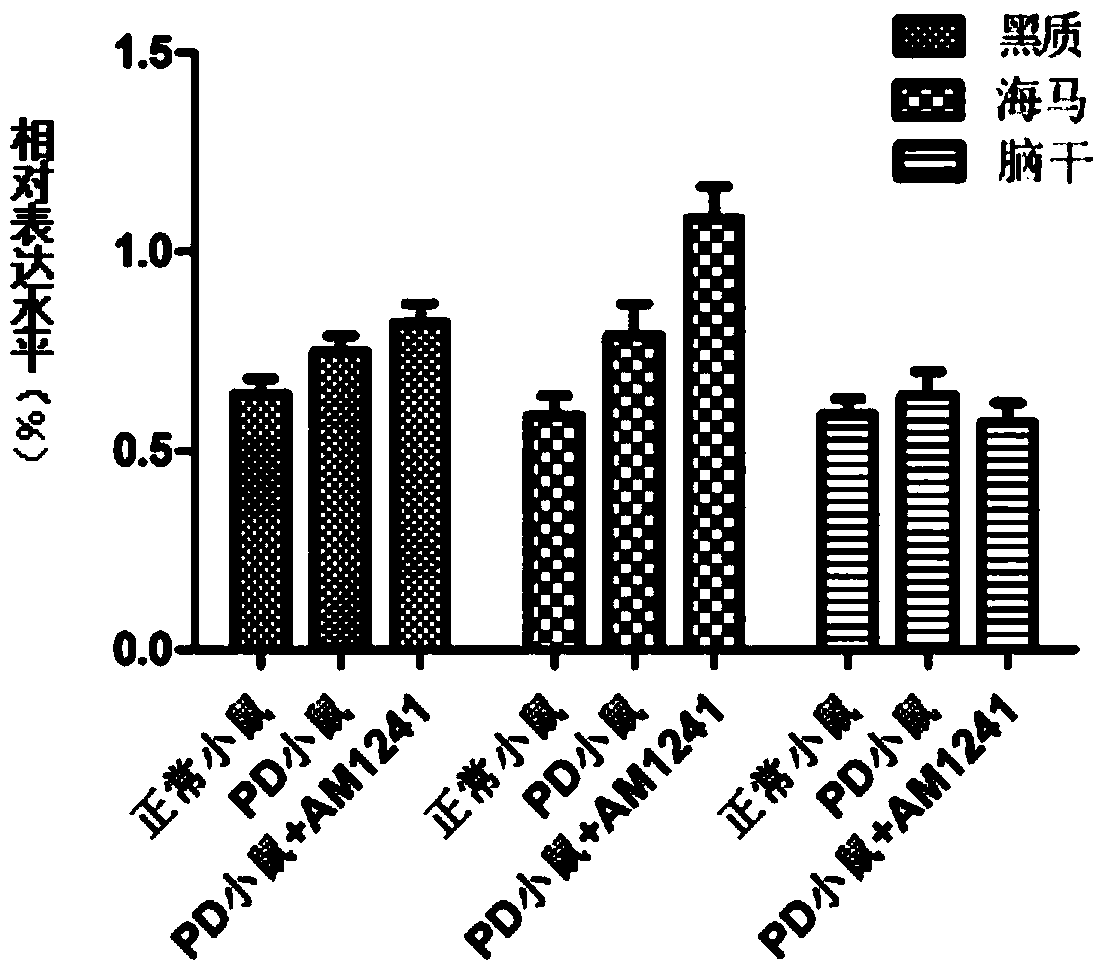
[0055] Example 3 Solid Lipid Nanoparticles (SLN-AM1241) of AM1241 Improves Brain Targeting Experiment
[0056] (1) Taking C57 mice with successful PD modeling as the experimental subjects, they were divided into groups and given AM1241 and SLN-AM1241 at a concentration of 1.5 mg / kg, and administered intraperitoneally for 14 consecutive days;
[0057] (2) The mice were killed on the 15th day, and each organ (heart, liver, spleen, lung, brain) was taken out respectively;
[0058] (3) Use tissue scissors or a scalpel to quickly separate the cleaned tissue into suitable small pieces, such as 1cm^3;
[0059] (4) Usually add 3 to 5 times the volume of pre-cooled homogenization buffer per volume of wet weight tissue to the homogenization container;
[0060] (5) Homogenize blade-type tissue breaker and incision-type tissue homogenizer at a speed of 1000-3000 rpm, high-speed homogenate 3 times, 20-30 seconds each time, pause for a few seconds between times, and operate on ice;
[006...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com