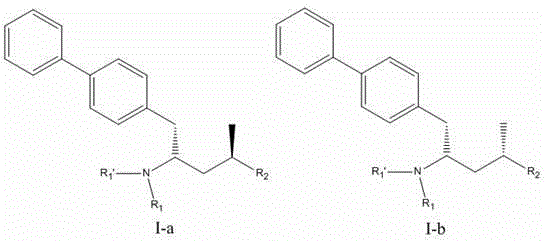
Preparation method and application of biaryl substituted 4-amino-butyric acid or derivative of biaryl substituted 4-amino-butyric acid
A carboxyl group and compound technology, which is applied in the application field of preparing NEP inhibitors, can solve the problems of low selectivity, low stability, and can not guarantee selectivity, etc., and achieves the effect of high stereoselectivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0041]
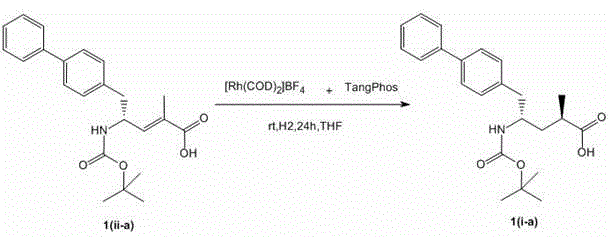
[0042] Add [Rh(COD) 2 ] BF 4 (315mg, 0.78mmol), the chiral phosphine ligand BICP (556mg, 0.84mmol) was added, the mixture was stirred for 30 minutes, 1(ii-a) (30g, 0.078mol), triethylamine (3.93g, 0.039 mmol). The hydrogenation was carried out at room temperature under hydrogen for 24 hours. After careful release of hydrogen gas, the reaction mixture was washed with methanol, then concentrated in vacuo. The residue was passed through a short silica gel column to remove the catalyst. Enantiomeric content was measured by capillary GC or HPLC. The reaction conversion rate is 100%, and the ee% is 99%.
[0043]
Embodiment 2
[0045]
[0046] Add [Rh(COD) 2 ] BF 4 (315mg, 0.78mmol), the chiral phosphine ligand BICPO (452mg, 0.84mmol) was added, and after the mixture was stirred for 30 minutes, 1(ii-a) (30g, 0.078mol) was added in sequence. The hydrogenation was carried out at room temperature under hydrogen for 24 hours. After careful release of hydrogen gas, the reaction mixture was washed with methanol, then concentrated in vacuo. The residue was passed through a short silica gel column to remove the catalyst. Enantiomeric content was measured by capillary GC or HPLC. The reaction conversion rate is 100%, and the ee% is 99%.
[0047]
Embodiment 3
[0049]
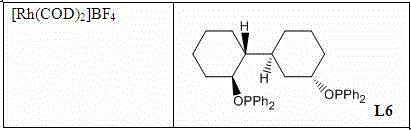
[0050] Add [Rh(COD) 2 ] BF 4 (315mg, 0.78mmol), chiral phosphine ligand L6 (475mg, 0.84mmol) was added, and after the mixture was stirred for 30 minutes, 1(ii-a) (30g, 0.078mol) was added in sequence. The hydrogenation was carried out at room temperature under hydrogen for 24 hours. After careful release of hydrogen gas, the reaction mixture was washed with methanol, then concentrated in vacuo. The residue was passed through a short silica gel column to remove the catalyst. Enantiomeric content was measured by capillary GC or HPLC. The reaction conversion rate is 100%, and the ee% is 96%.
[0051]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com