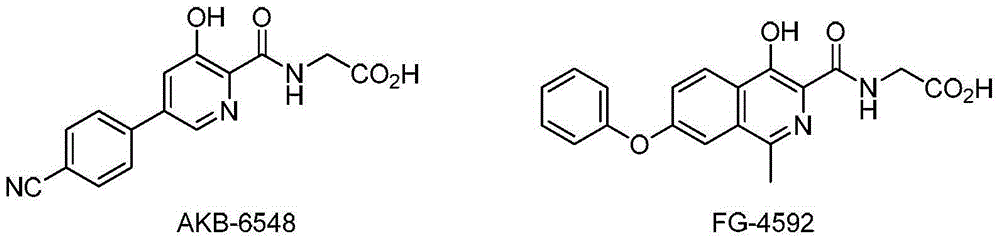
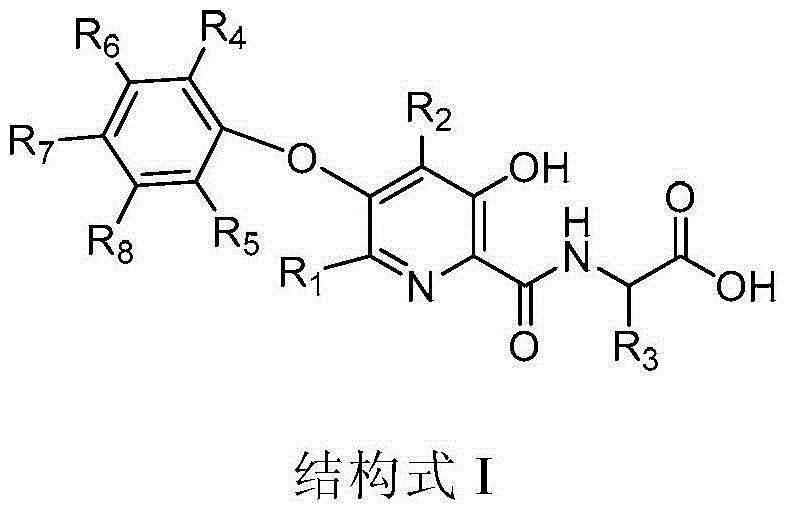
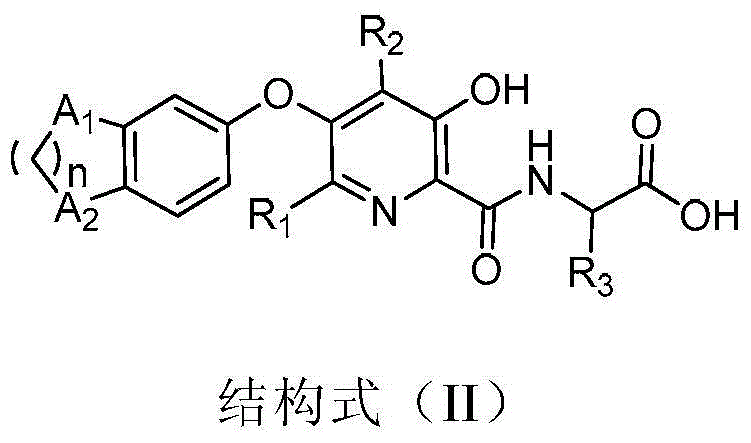
3-hydroxypyridine compound and its preparation method and pharmaceutical use
一种化合物、药学的技术,应用在医药领域,能够解决半衰期短、病患大负担、EPO使用费用高等问题
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0053]
[0054] 2-(3-Hydroxy-5-phenoxy-2-pyridinecarboxamido)acetic acid (Compound No. 1)
[0055] Step 1: Preparation of 5-bromo-3-methoxypyridine-2-carbonitrile
[0056] A solution of sodium methoxide (9.7 g, 0.18 mol) in methanol (50 ml) was added dropwise to a suspension of 5-bromo-3-fluoropyridine-2-carbonitrile (30 g, 0.15 mol) in methanol (150 ml) at room temperature. After the dropwise addition was completed, the reaction solution became clear after reacting at room temperature for 2 hours. Add a small amount of glacial acetic acid to the reaction solution to adjust the pH to 7-8, add ice water (300ml), concentrate the reaction solution until solids precipitate, then cool and stand for 2 hours to make the solids precipitate more thoroughly. The precipitated solid was suction-filtered, the filter cake was washed with water, and the filter cake was collected and air-dried at room temperature to obtain a white solid 5-bromo-3-methoxypyridine-2-carbonitrile (24 g, yiel...
Embodiment 2
[0066]
[0067] 2-(5-(2,3-Dimethylphenoxy)-3-hydroxypyridine-2-carboxamido)acetic acid (Compound No. 5)
[0068] Step 1: Preparation of 5-bromo-3-methoxypyridine-2-carbonitrile
[0069] A solution of sodium methoxide (9.7 g, 0.18 mol) in methanol (50 ml) was added dropwise to a suspension of 5-bromo-3-fluoropyridine-2-carbonitrile (30 g, 0.15 mol) in methanol (150 ml) at room temperature. After the dropwise addition was completed, the reaction solution became clear after reacting at room temperature for 2 hours. Add a small amount of glacial acetic acid to the reaction solution to adjust the pH to 7-8, add ice water (300ml), concentrate the reaction solution until solids precipitate, then cool and stand for 2 hours to make the solids precipitate more thoroughly. The precipitated solid was suction-filtered, the filter cake was washed with water, and the filter cake was collected and air-dried at room temperature to obtain a white solid 5-bromo-3-methoxypyridine-2-carbonitri...
Embodiment 3
[0079]
[0080] 2-(5-(3-Chlorophenoxy)-3-hydroxypyridine-2-carboxamido)acetic acid (Compound No. 22)
[0081] Step 1: Preparation of 5-bromo-3-methoxypyridine-2-carbonitrile
[0082] A solution of sodium methoxide (9.7 g, 0.18 mol) in methanol (50 ml) was added dropwise to a suspension of 5-bromo-3-fluoropyridine-2-carbonitrile (30 g, 0.15 mol) in methanol (150 ml) at room temperature. After the dropwise addition was completed, the reaction solution became clear after reacting at room temperature for 2 hours. Add a small amount of glacial acetic acid to the reaction solution to adjust the pH to 7-8, add ice water (300ml), concentrate the reaction solution until solids are precipitated, then cool and stand for 2 hours to make the solids precipitate more thoroughly. The precipitated solid was suction-filtered, the filter cake was washed with water, and the filter cake was collected and air-dried at room temperature to obtain a white solid 5-bromo-3-methoxypyridine-2-carbonit...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com