Method for preparing lurasidone with high purity and high yield
A lurasidone, high-yield technology, applied in the field of preparation of pharmaceutical compounds, can solve the problems of easy generation of impurities, shortened reaction time, and lack of quality of patents, and achieve the effect of controlling reaction impurities and reducing reaction reflux temperature
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
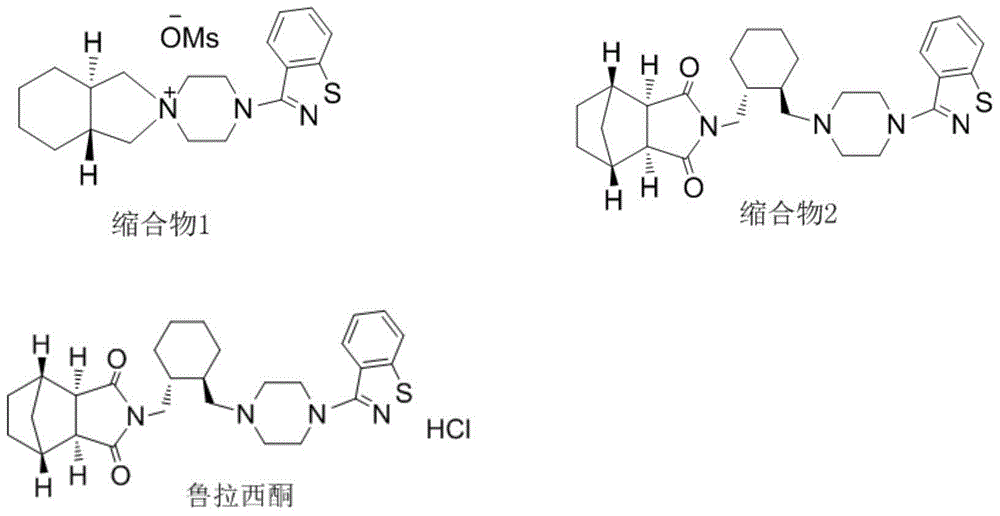
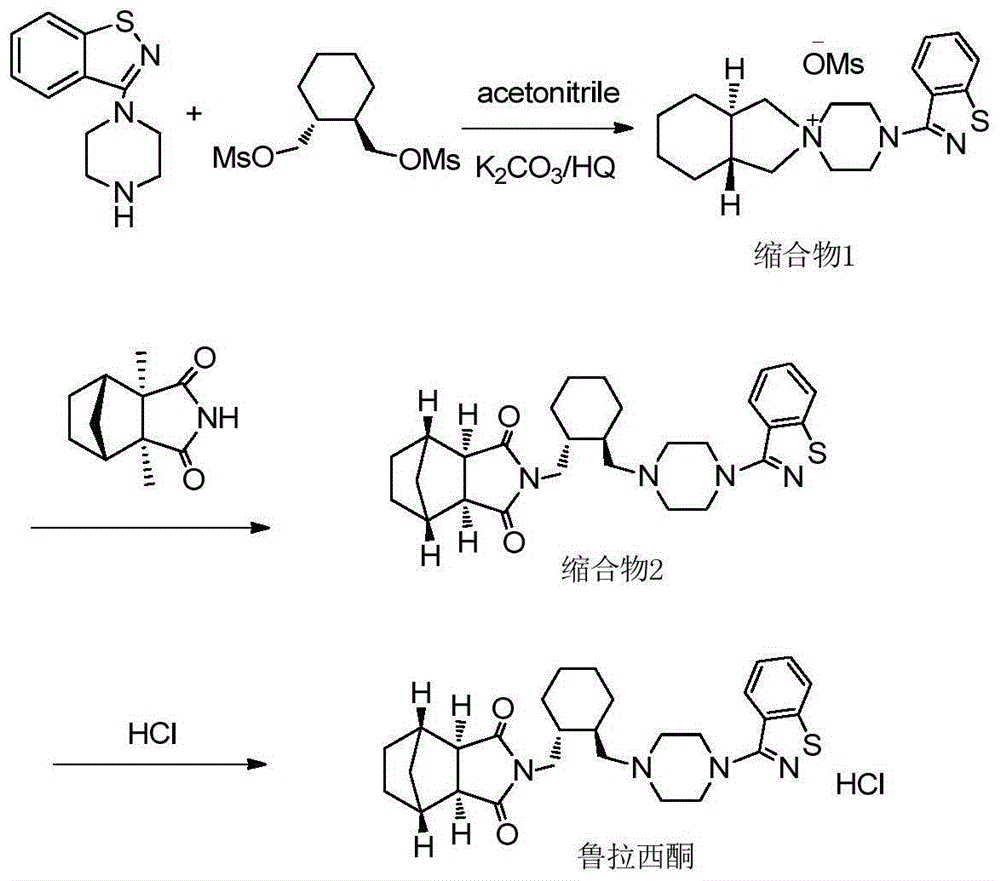
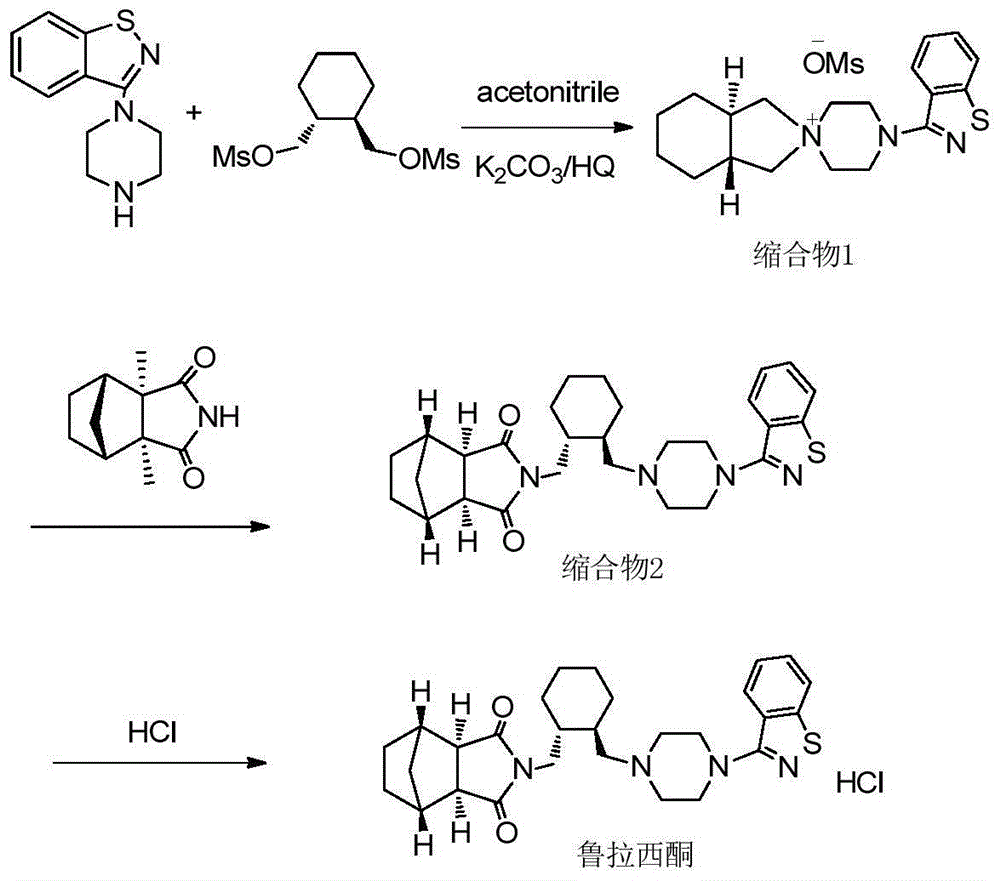
[0028] 6g (20mmol) (1R, 2R)-1,2-bis(methanesulfonate oxymethyl)cyclohexane and 4.9g (22.3mmol) 3-(1-piperazinyl)-1 were put into the reaction flask, Add 2-benzisothiazole and 3.5g (25.3mmol) of potassium carbonate, add 50ml of acetonitrile and 1.5g (83mmol) of water, heat to reflux (82°C) for 3 hours, cool to room temperature after the reaction, filter to remove inorganic salts, the solution reduces Concentrate under reduced pressure until the solvent is exhausted, add 25ml of ethyl acetate, stir and crystallize, filter at 5°C, and dry in vacuo to obtain 7.7g of white crystals (condensate 1) (91% yield, HPLC≥98.0%).
[0029] Put 7.7g (18.2mmol) condensate 1 and 4.5g (32.6mmol) potassium carbonate and 60ml toluene in the reaction flask, stir for 5min, add 3.2g (19.4mmol) (3aR, 4S, 7R, 7aS) 4,7- Methylene-1H-isoindole-1,3(2H)-dione, heated to reflux (108°C) for 3 hours, cooled to room temperature after the reaction, added 50ml of water and stirred, then stood still, separated to...
Embodiment 2
[0031] 6g (20mmol) (1R, 2R)-1,2-bis(methanesulfonate oxymethyl)cyclohexane and 4.9g (22.3mmol) 3-(1-piperazinyl)-1 were put into the reaction flask, Add 2-benzisothiazole and 3.5g (25.3mmol) potassium carbonate, add 45ml acetonitrile, 2g (62.5mmol) methanol and 1g (55.5mmol) water, heat to reflux (78°C) for 3h, cool to room temperature after the reaction, Filter to remove inorganic salts, concentrate the solution under reduced pressure until the solvent is exhausted, add 25ml of ethyl acetate to stir and crystallize, filter at 5°C, and vacuum-dry to obtain 7.6g of white crystals (condensate 1) (yield 89.8%, HPLC≥97.5%) .
[0032] Put 7.6g (17.9mmol) condensate 1 and 4.5g (32.6mmol) potassium carbonate and 60ml toluene in the reaction flask, stir for 5min, add 3.2g (19.4mmol) (3aR, 4S, 7R, 7aS) 4,7- Methylene-1H-isoindole-1,3(2H)-dione, heated to reflux (108°C) for 3 hours, cooled to room temperature after the reaction, added 50ml of water and stirred, then stood still, separa...
Embodiment 3
[0035] 6g (20mmol) (1R, 2R)-1,2-bis(methanesulfonate oxymethyl)cyclohexane and 4.9g (22.3mmol) 3-(1-piperazinyl)-1 were put into the reaction flask, Add 2-benzisothiazole and 3.5g (25.3mmol) potassium carbonate, add 50ml acetonitrile and 3.2g (100mmol) methanol, heat to reflux (75°C) for 3.5h, cool to room temperature after the reaction, filter to remove inorganic salts, the solution Concentrate under reduced pressure until the solvent is exhausted, add 25ml of ethyl acetate, stir and crystallize, filter at 5°C, and dry in vacuo to obtain 7.4g of white crystals (condensate 1) (yield 87.5%, HPLC≥95.0%).
[0036] Put 7.4g (17.5mmol) condensate 1 and 4.4g (31.9mmol) potassium carbonate and 60ml toluene in the reaction flask, stir for 5min, add 3.1g (18.8mmol) (3aR, 4S, 7R, 7aS) 4,7- Methylene-1H-isoindole-1,3(2H)-dione, heated to reflux (108°C) for 3 hours, cooled to room temperature after the reaction, added 50ml of water and stirred, then stood still, separated to remove the wa...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com