A kind of synthetic method of 5-bromo-7-azaindole
A kind of technology of azaindole and synthesis method, applied in the field of synthesis of 5-bromo-7-azaindole, can solve the problems of long reaction time, low economic benefit, complicated reaction operation, etc., achieve shortening the time of reaction, Optimized reaction conditions and high reaction selectivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0075] A synthetic method of 5-bromo-7-azaindole, comprising the following steps:
[0076] The first step: synthesis of 7-azaindoline:
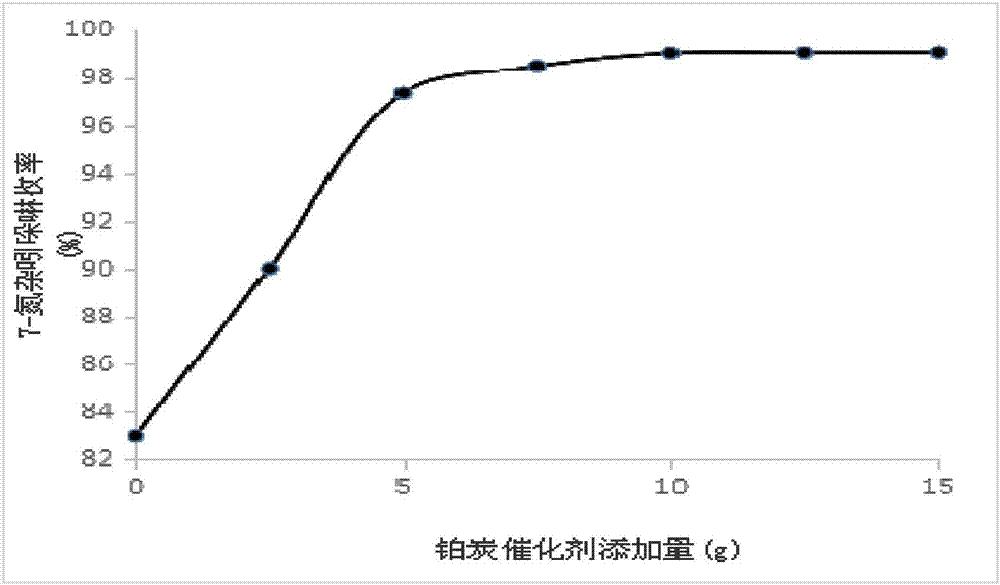
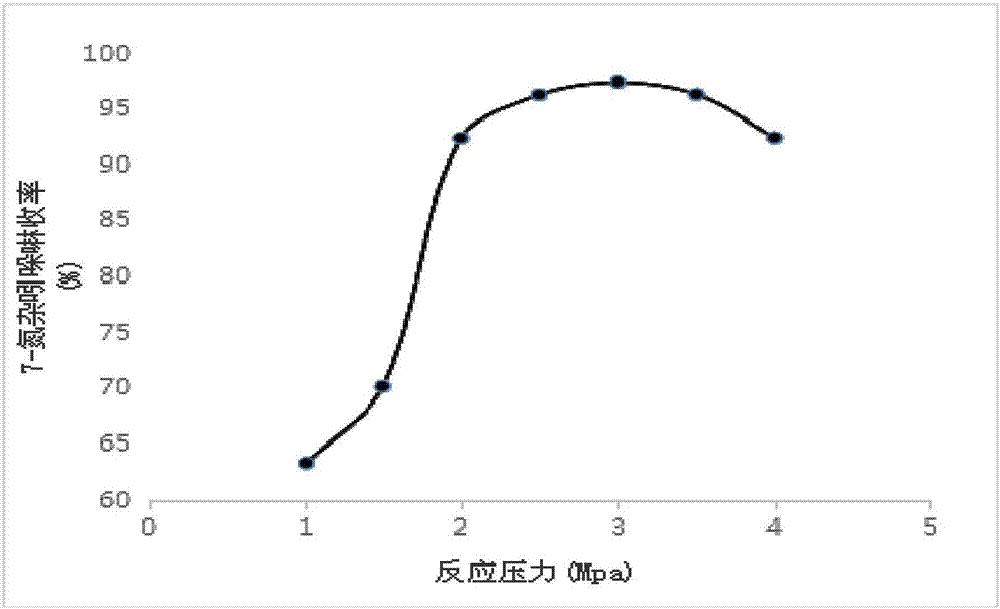
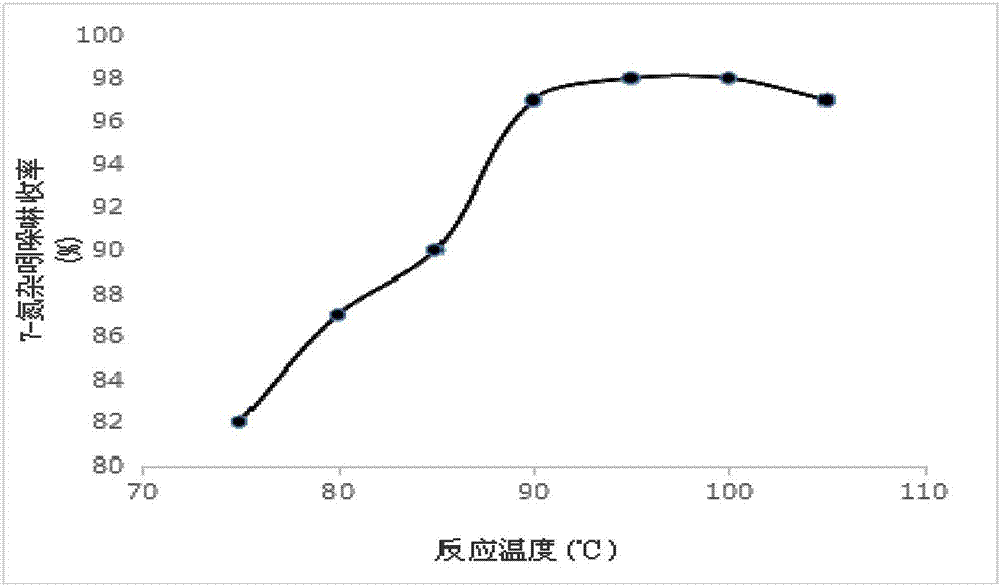
[0077] Add 30g of 7-azaindole and 5g of platinum carbon catalyst (3% Pt content) into the pressure reactor, add 250mL of cyclohexane as a solvent, fill in 2MPa hydrogen with stirring, raise the temperature to 90°C, and react for 5h ;
[0078] After cooling and filtering, the filter cake was washed twice with cyclohexane, the filtrate was combined, the solvent was concentrated and evaporated to dryness to obtain 7-azaindoline;
[0079] The reaction in this step is a light-avoiding reaction;
[0080] The second step: Synthesis of 5-bromo-7-azaindoline:
[0081] Add the 7-azaindoline, 300mL of dichloromethane, and 3g of sodium bromide synthesized in the first step above into a 1L round-bottomed flask, mix and stir, add 48g of bromine dropwise at room temperature, and finish the dropwise addition in 30 minutes. After the addition, the tempera...
Embodiment 2
[0088] A synthetic method of 5-bromo-7-azaindole, comprising the following steps:
[0089] The first step: synthesis of 7-azaindoline:
[0090] Add 30g of 7-azaindole and 10g of platinum carbon catalyst (5% Pt content) into the pressure reactor, add 250mL of cyclohexane as a solvent, fill with 3MPa hydrogen under stirring, raise the temperature to 95°C, and react for 8h ;
[0091] After cooling and filtering, the filter cake was washed twice with cyclohexane, the filtrate was combined, the solvent was concentrated and evaporated to dryness to obtain 7-azaindoline;
[0092] The reaction in this step is a light-avoiding reaction;
[0093] The second step: Synthesis of 5-bromo-7-azaindoline:
[0094] Add the 7-azaindoline, 300mL of dichloromethane, and 4g of sodium bromide synthesized in the first step above into a 1L round-bottomed flask, mix and stir, add 50g of bromine dropwise at room temperature, and finish the dropwise addition in 30 minutes. After the addition, the tem...
Embodiment 3
[0101] A synthetic method of 5-bromo-7-azaindole, comprising the following steps:
[0102] The first step: synthesis of 7-azaindoline:
[0103] 30g of 7-azaindole and 5g of platinum carbon catalyst (5% Pt content) were added to the pressure reactor, 250mL of cyclohexane was added as a solvent, and 2-3MPa hydrogen gas was charged under stirring, and the temperature was raised to 90°C. Reaction 8h;
[0104] After cooling and filtering, the filter cake was washed twice with cyclohexane, the filtrate was combined, the solvent was concentrated and evaporated to dryness to obtain 7-azaindoline;
[0105] The reaction in this step is a light-avoiding reaction;
[0106] The second step: Synthesis of 5-bromo-7-azaindoline:
[0107] Add 7-azaindoline, 300mL of dichloromethane, and 3g of sodium bromide synthesized in the first step above into a 1L round-bottomed flask, mix and stir, and add 50g of bromine dropwise at room temperature, and the dropwise addition is completed in 30 minute...
PUM
| Property | Measurement | Unit |
|---|---|---|
| specific surface area | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com