Synthesis method of Ramosetron
A technology of ramosetron and a synthetic method, which is applied in the field of chemical synthesis, can solve the problems of low economic benefit and low yield, and achieve the effects of reducing reaction activation energy, improving economic benefit, and increasing reaction yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
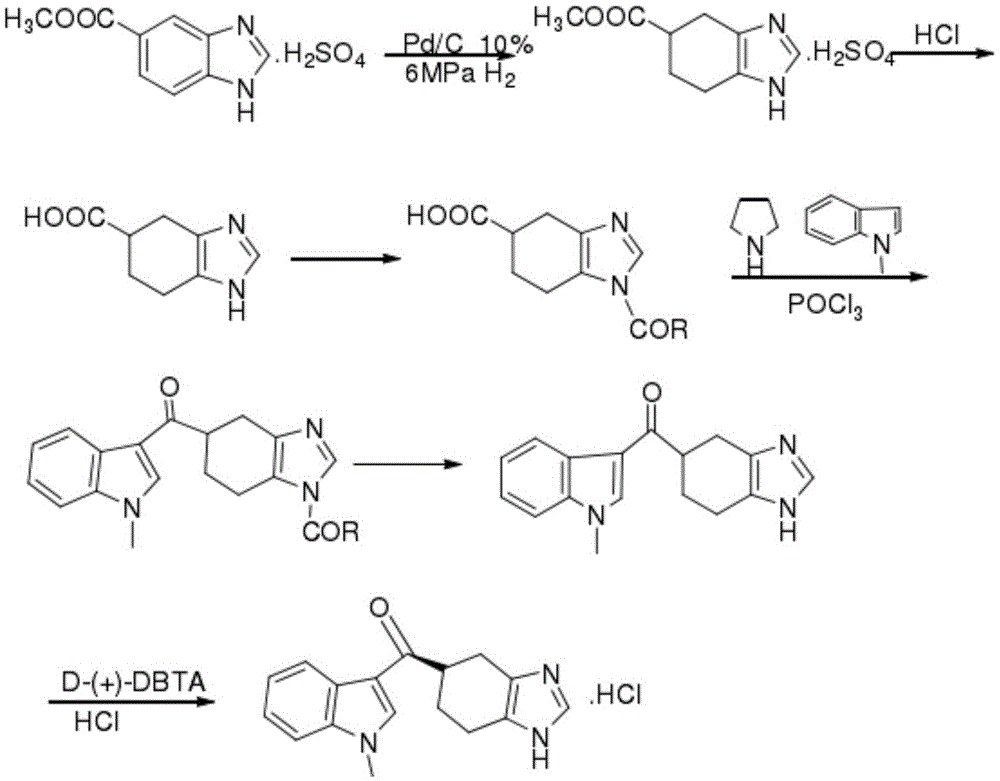
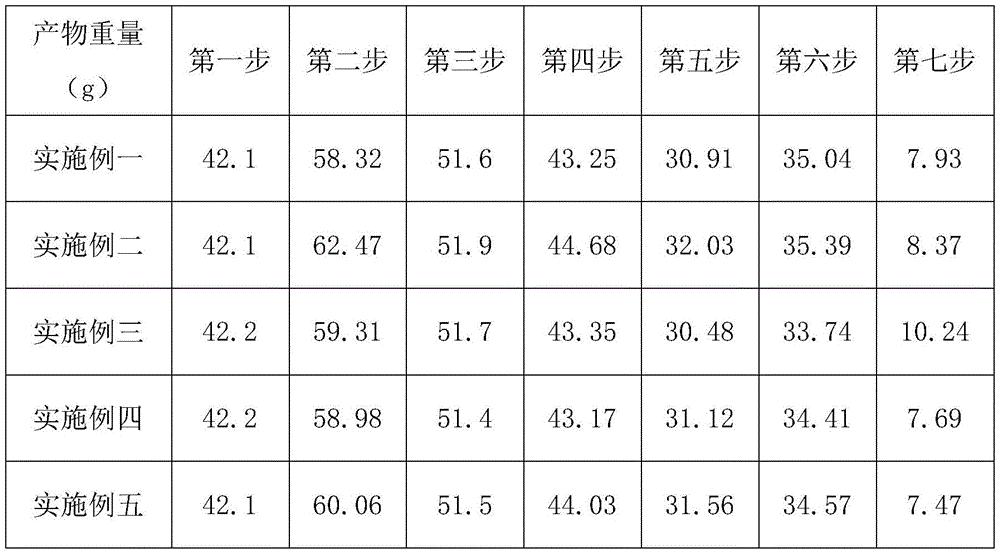
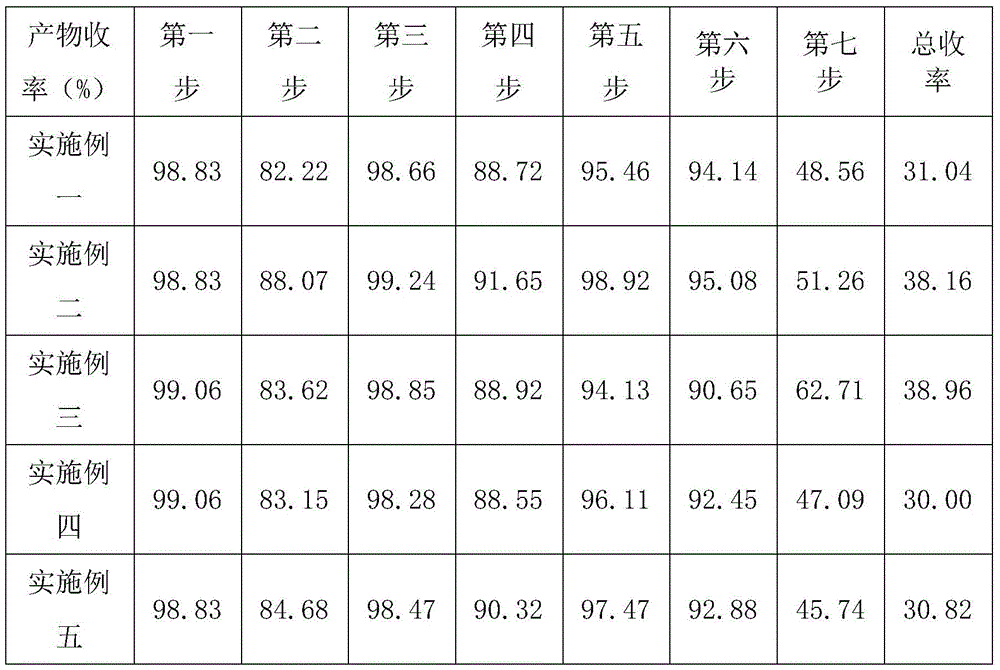
[0043] A synthetic method for ramosetron, using 3,4-diaminobenzoic acid as a starting material, through seven steps of synthesizing ramosetron, comprising the following steps:
[0044] Step 1: Synthesis of benzimidazole-5-carboxylic acid
[0045] Add 500ml of formic acid to 40g of 3,4-diaminobenzoic acid, stir and heat to reflux for 4h, concentrate under reduced pressure, add 700ml of water, adjust the pH to 5-6 with ammonia water, filter and dry to obtain off-white solid benzimidazole-5-carboxylic acid;
[0046] The second step: synthesis of benzimidazole-5-carboxylate methyl sulfate
[0047] Add 500ml of anhydrous methanol to the benzimidazole-5-carboxylic acid prepared in the first step, add 45ml of thionyl chloride dropwise at a temperature of 7-8°C, and raise the temperature to 50-52°C for reflux reaction for 5h after dropping, then reflux reaction After cooling down to room temperature, add 800ml of water, adjust the pH to 8-9 with ammonia water, filter and dry to obtai...
Embodiment 2
[0059] A synthetic method for ramosetron, using 3,4-diaminobenzoic acid as a starting material, through seven steps of synthesizing ramosetron, comprising the following steps:
[0060] Step 1: Synthesis of benzimidazole-5-carboxylic acid
[0061] Add 500ml of formic acid to 40g of 3,4-diaminobenzoic acid, stir and heat to reflux for 4h, concentrate under reduced pressure, add 700ml of water, adjust the pH to 5-6 with ammonia water, filter and dry to obtain off-white solid benzimidazole-5-carboxylic acid;
[0062] The second step: synthesis of benzimidazole-5-carboxylate methyl sulfate
[0063] Add 500ml of anhydrous methanol to the benzimidazole-5-carboxylic acid prepared in the first step, add 45ml of thionyl chloride dropwise at 8°C, raise the temperature to 50°C for reflux reaction for 5h after the dropwise reaction, and cool down to room temperature after the reflux reaction Add 800ml of water, adjust the pH to 9 with ammonia water, filter and dry to obtain a taupe solid;...
Embodiment 3
[0075] A synthetic method for ramosetron, using 3,4-diaminobenzoic acid as a starting material, through seven steps of synthesizing ramosetron, comprising the following steps:
[0076] Step 1: Synthesis of benzimidazole-5-carboxylic acid
[0077] Add 500ml of formic acid to 40g of 3,4-diaminobenzoic acid, stir and heat to reflux for 4h, concentrate under reduced pressure, add 700ml of water, adjust the pH to 5-6 with ammonia water, filter and dry to obtain off-white solid benzimidazole-5-carboxylic acid;
[0078] The second step: synthesis of benzimidazole-5-carboxylate methyl sulfate
[0079] Add 500ml of anhydrous methanol to the benzimidazole-5-carboxylic acid prepared in the first step, add 45ml of thionyl chloride dropwise at a temperature of 7-8°C, raise the temperature to 53-55°C for reflux reaction for 5 hours after dropping, and then reflux reaction After cooling down to room temperature, add 800ml of water, adjust the pH to 8-9 with ammonia water, filter and dry to ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com