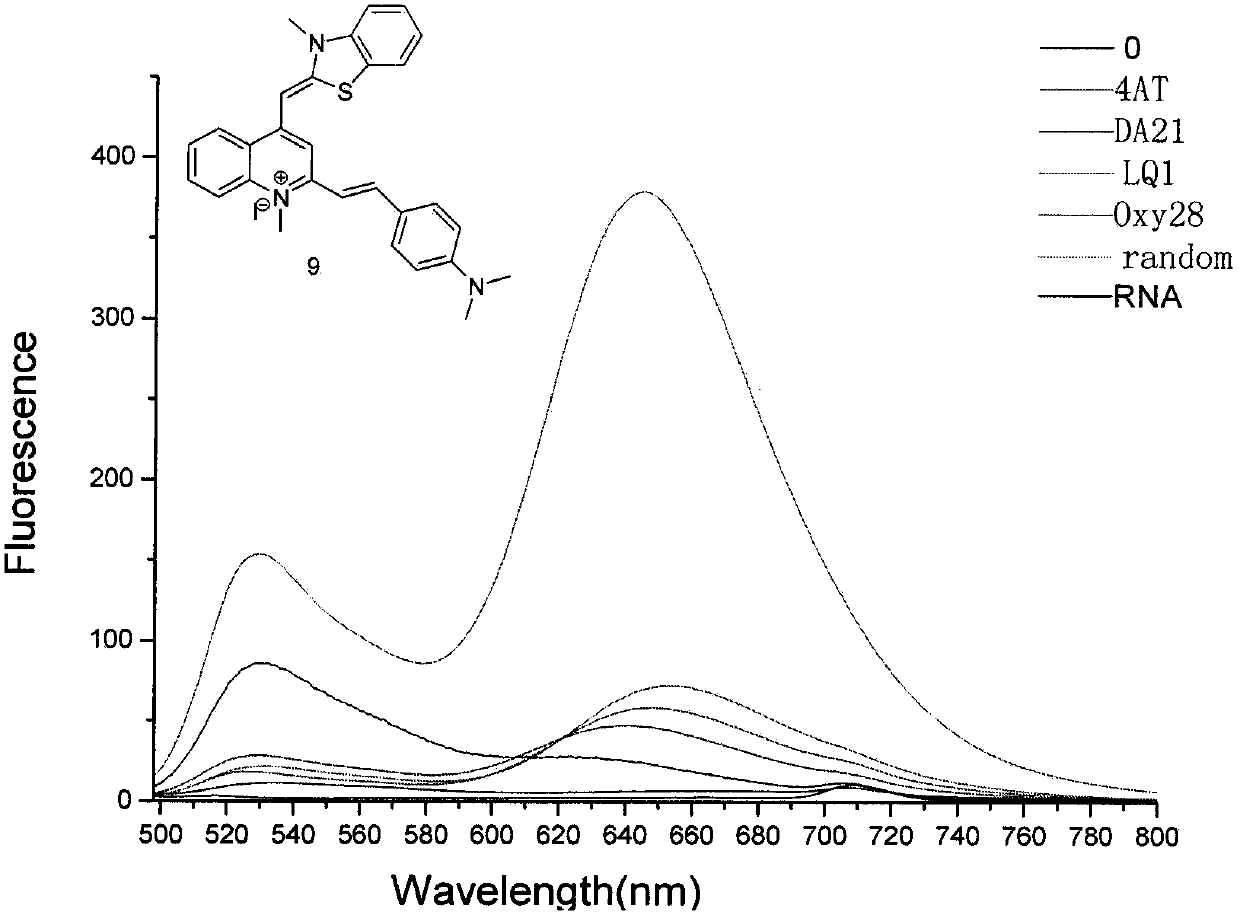
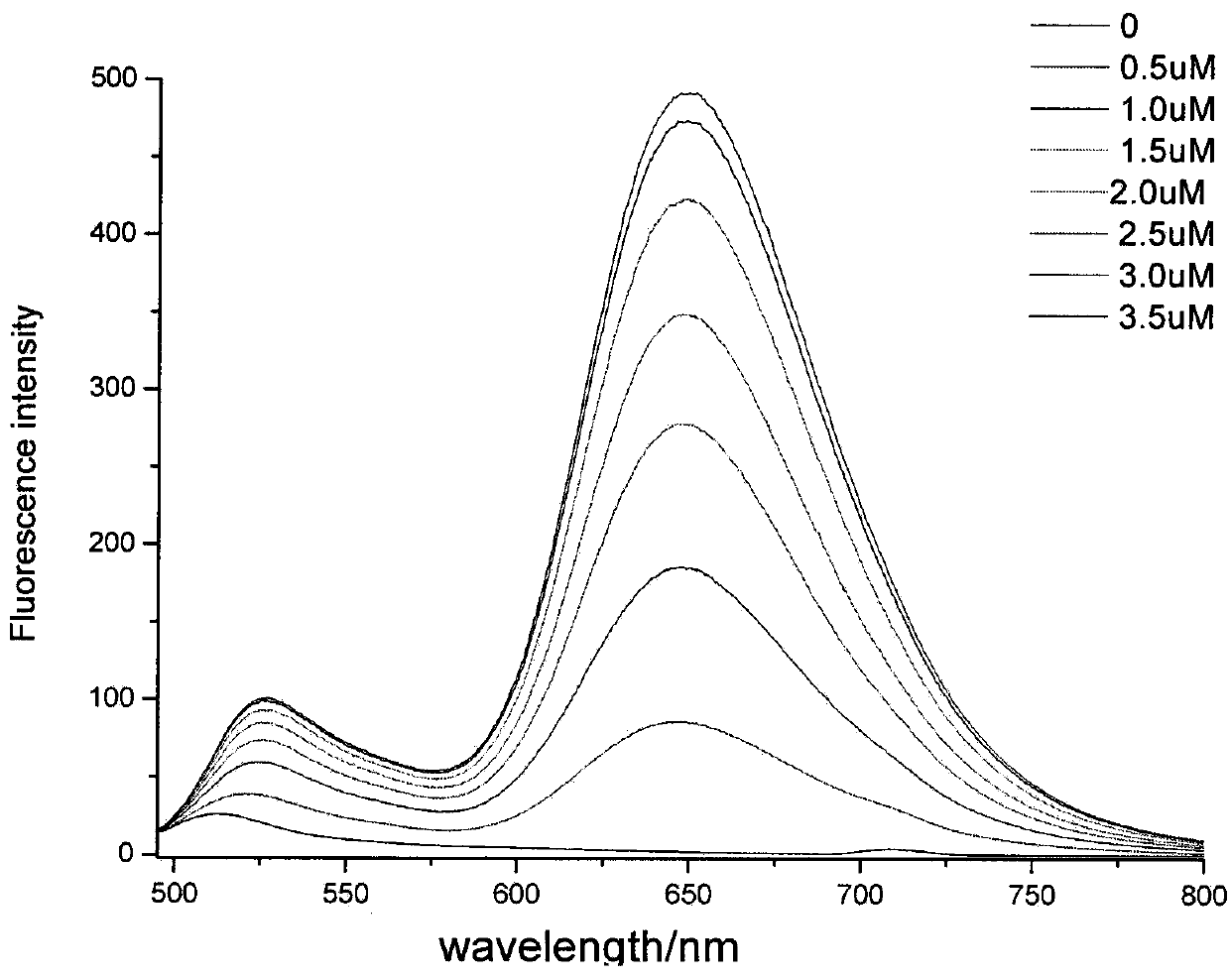
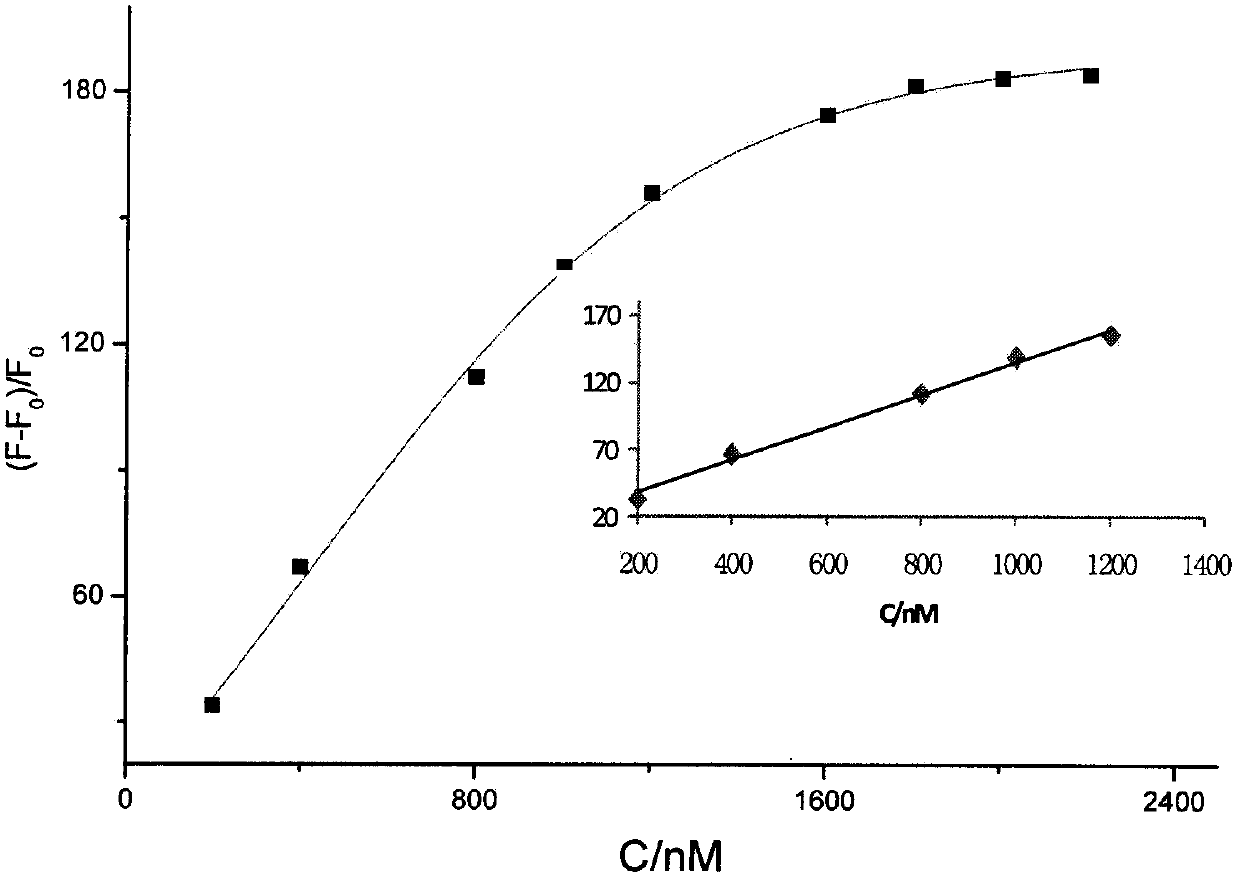
Thiazole orange styrenics as fluorescent probes for g-quadruplex nucleic acids
A technology of fluorescent probes and quadruplexes, applied in fluorescence/phosphorescence, organic chemistry, luminescent materials, etc., can solve the problems of poor selectivity limiting the application of TO, failure to recognize the secondary structure of G-quadruplexes, etc., and achieve a good cell membrane Permeability, good photostability, simple preparation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] Embodiment one: the synthesis of compound 2
[0036] Weigh 0.2g (1.1236mmol) of 4-chloro-quinaldine into a 25ml round bottom flask, add methyl iodide and sulfolane as a solvent, heat the mixture to 40-60°C, react for 18 hours, cool, and add anhydrous diethyl ether After shaking, suction filtration, the solid was washed several times, weighed after vacuum drying, and thin layer chromatography showed that there were no by-products, and 0.345g of pure product 2 was obtained, with a yield of 95.8%: 1 H NMR (400MHz, DMSO) δ8.56(d, J=8.4Hz, 1H), 8.46(d, J=8.3Hz, 1H), 8.22(t, J=8.1Hz, 1H), 8.01(t, J =7.9Hz, 1H), 7.55(s, J=7.4Hz, 1H), 4.20(s, 3H), 3.74(s, 1H), 2.68(s, 3H).
Embodiment 2
[0037] Embodiment two: the synthesis of compound 4
[0038] Weigh 0.25g (1.68mmol) of 2-methyl-benzothiazole into a 25ml round bottom flask, add methyl iodide and absolute ethanol as a solvent, and react at 80°C for 15 hours, then cool the reacted solution to room temperature , then add the mixed solution of absolute ethanol and chloroform, shake and filter with suction, and wash the precipitate with a small amount of reagent, and obtain 0.448g of white powdery solid after vacuum drying, the yield is 91.7%: 1H NMR (400MHz, DMSO) δ8.44(d, J=8.1Hz, 1H), 8.30(d, J=8.4Hz, 1H), 7.90(t, J=7.8Hz, 1H), 7.81(t, J =7.7Hz, 1H), 4.20(s, 3H), 3.54(s, 1H), 3.17(s, 3H).
Embodiment 3
[0039] Embodiment three: the synthesis of compound 5
[0040] Weigh 0.50 g each of compounds 2 and 3, add them to a round-bottomed flask containing 10 ml of methanol, stir at room temperature, add a small amount of 0.5 mol / L sodium bicarbonate aqueous solution, and stir for about 1 hour. Add 4ml of saturated KI solution to the reacted solution, stir for about 5 minutes, then filter with suction, then wash with water and acetone to finally obtain a brick-red solid, which is dried, filtered, concentrated, and purified by column chromatography to obtain 0.98g of compound 4. The rate is 81.7%: 1 H NMR (400MHz, DMSO) δ8.77(d, J=8.3Hz, 1H), 8.18(d, J=8.7Hz, 1H), 8.02-7.96(m, 2H), 7.74(d, J=8.2Hz , 2H), 7.59(t, J=7.7Hz, 1H), 7.39(t, J=7.5Hz, 1H), 7.34(s, 1H), 6.85(s, 1H), 4.07(s, 3H), 3.98 (s,3H), 2.87(s,3H).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com