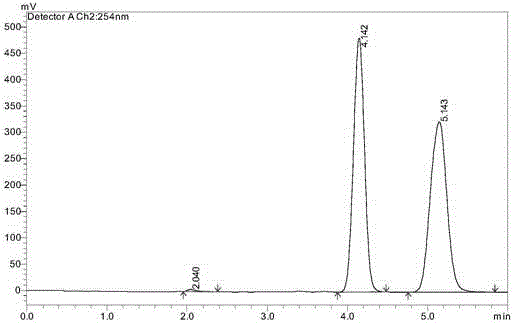
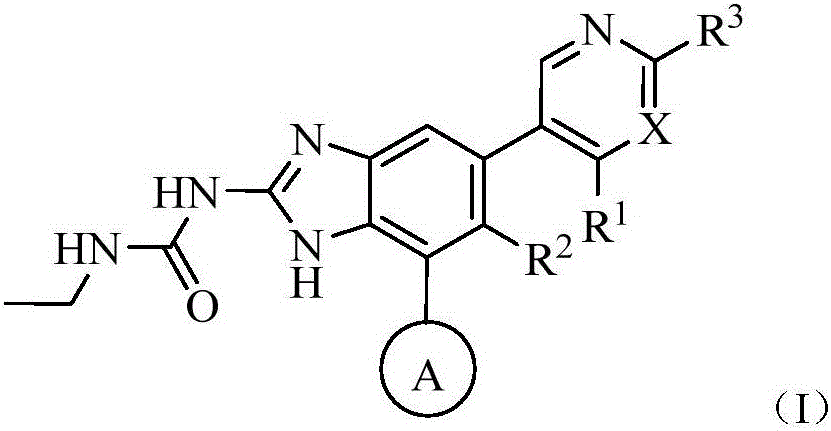
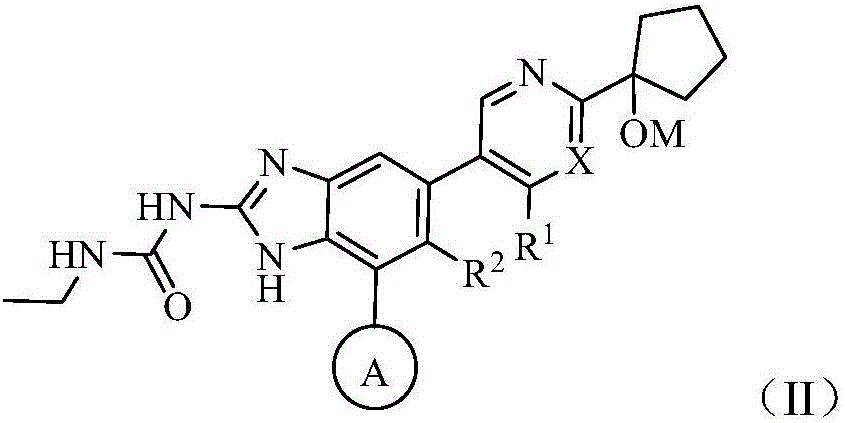
Monocycle gyrase and topoisomerase IV inhibitor
A technology of stereoisomers and heterocyclic groups, applied in medical preparations containing active ingredients, organic active ingredients, organic chemistry, etc., can solve problems such as poor solubility, poor penetration of bacterial outer membrane, and lack of clinical application
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0124] The present invention also provides the preparation method of above-mentioned compound:
[0125]
[0126] Reaction steps:
[0127] (1) Preparation of intermediate 1
[0128] Add raw materials 2, 1,4-dioxane, BiPin to the reaction flask 2 , KAc, palladium catalyst (such as Pd(dppf)Cl 2 ) and halogenated organic solvents (such as dichloromethane, chloroform), heating under nitrogen protection, cooling, adding sodium bicarbonate, water, raw material 1, palladium catalyst (such as Pd(dppf)Cl 2 ) and halogenated organic solvents (such as dichloromethane, chloroform), continue to react for about 4h. The reaction solution was concentrated and purified to obtain Intermediate I. Y represents halogen.
[0129] (2) Preparation of Intermediate 2
[0130] Add intermediate 1 into a mixed organic solvent (such as methanol, ethanol, tetrahydrofuran, triethylamine), add palladium on carbon, and stir under hydrogen atmosphere until the reaction is complete. Suction filtration a...
experiment example 1
[0169] The in vitro antibacterial activity of experimental example 1 compound of the present invention
[0170] Tested strains: All the clinically isolated strains used in the test were purchased from public institutions.
[0171] LRE and LRS strains were obtained from Peking University First Hospital; MRSA, MRSE, PRSP and other strains were purchased from Jinan Central Hospital, Renji Hospital Affiliated to Shanghai Jiao Tong Fourth Hospital and the Affiliated Hospital of the Third Military Medical University.
[0172] Test items: compounds 1, 2, 3, 15, 17, 20, 55, 56, 57, 59;
[0173] Compound of the present invention, its chemical name and structural formula see embodiment;
[0174] Experimental method: agar dilution method, refer to National Committee for Clinical Laboratory Standards.2006.Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria That Grow Aerobically; Approved Standard--Seventh Edition M7-A7.Vol26, no.2, Wayne, PA: Clinical And Laboratory S...
experiment example 2
[0183] Rat Pharmacokinetic Experiment of Experimental Example 2 Compound of the present invention
[0184] Test samples: Compound 2, Compound 20, Compound 55, and Compound 57 of the present invention are self-made, and their chemical names and preparation methods are shown in the preparation examples of each compound.
[0185] Test animals: male SD rats, 3 / administration dose / test product, body weight 210-290g / rat.
[0186] Preparation of the test solution:
[0187] Preparation of blank solvent:
[0188] Preparation of 28% Captisol solution: Weigh 8.4g of Captisol, add a small amount of purified water to dissolve it ultrasonically, then dilute to 30mL with purified water, vortex and mix to obtain the product.
[0189] Preparation of 28% and 40% HP-β-CD solutions: Weigh 2.8g and 4.0g of HP-β-CD, prepare 10mL solutions with sterilized water for injection, and vortex to obtain the solution.
[0190](1) Compound 2, intravenous injection (iv) prescription: 5% DMSO+10% PEG400+85%...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com