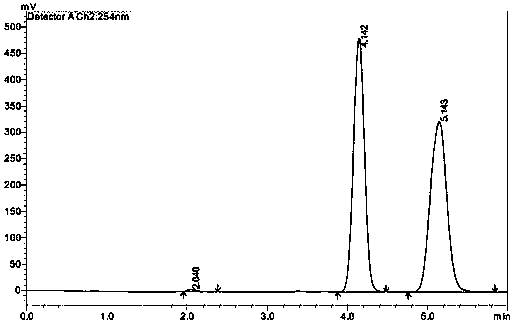
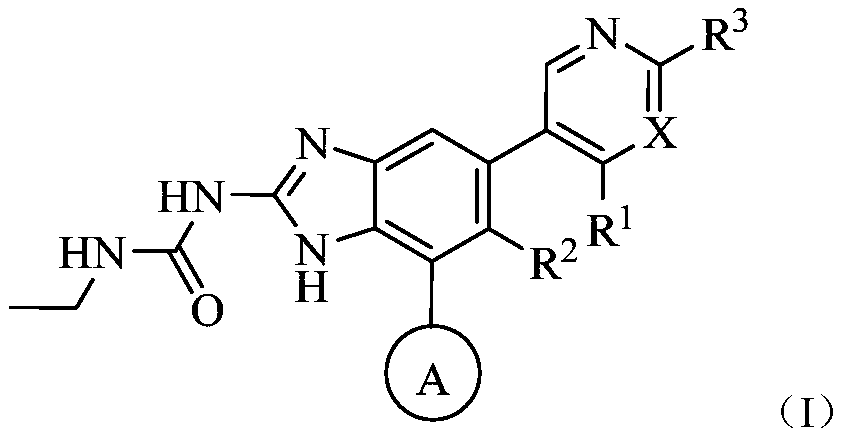
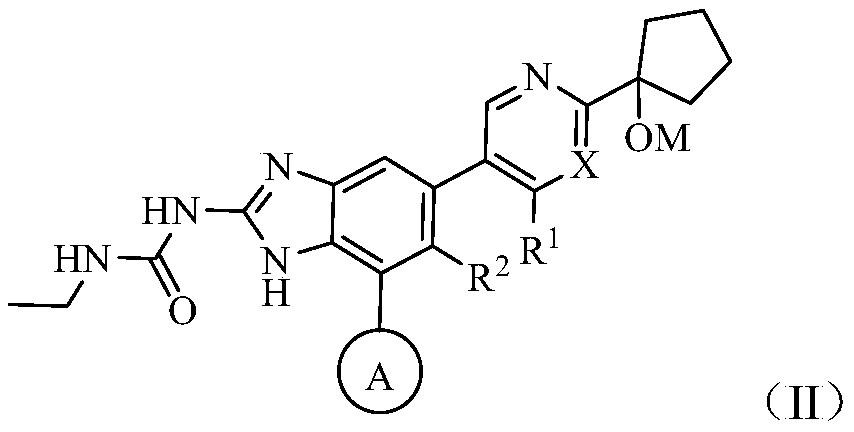
Monocyclic gyrase and topoisomerase IV inhibitors
A stereoisomer, selected technology, applied in the field of medicine, can solve the problems of poor bacterial outer membrane penetration, poor solubility, and no clinical application
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0124] The present invention also provides the preparation method of above-mentioned compound:
[0125]
[0126] Reaction steps:
[0127] (1) Preparation of Intermediate 1
[0128] Add raw materials 2, 1,4-dioxane, BiPin to the reaction flask 2 , KAc, palladium catalyst (such as Pd(dppf)Cl 2 ) and halogenated organic solvents (such as dichloromethane, chloroform), heating under nitrogen protection, cooling, adding sodium bicarbonate, water, raw material 1, palladium catalyst (such as Pd(dppf)Cl 2 ) and halogenated organic solvents (such as dichloromethane, chloroform), continue to react for about 4h. The reaction solution was concentrated and purified to obtain Intermediate I. Y represents halogen.
[0129] (2) Preparation of Intermediate 2
[0130] Add intermediate 1 into a mixed organic solvent (such as methanol, ethanol, tetrahydrofuran, triethylamine), add palladium on carbon, and stir under hydrogen atmosphere until the reaction is complete. Suction filtration a...
experiment example 1
[0169] The in vitro antibacterial activity of experimental example 1 compound of the present invention
[0170] Tested strains: All the clinically isolated strains used in the test were purchased from public institutions.
[0171] LRE and LRS strains were obtained from Peking University First Hospital; MRSA, MRSE, PRSP and other strains were purchased from Jinan Central Hospital, Renji Hospital Affiliated to Shanghai Jiao Tong Fourth Hospital and the Affiliated Hospital of the Third Military Medical University.
[0172] Test items: compounds 1, 2, 3, 15, 17, 20, 55, 56, 57, 59;
[0173] Compound of the present invention, its chemical name and structural formula see embodiment;
[0174] Experimental method: agar dilution method, refer to National Committee for Clinical Laboratory Standards.2006.Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria That Grow Aerobically; Approved Standard--Seventh Edition M7-A7.Vol26, no.2, Wayne, PA: Clinical And Laboratory S...
experiment example 2
[0183] Rat Pharmacokinetic Experiment of Experimental Example 2 Compound of the present invention
[0184] Test products: Compound 2, Compound 20, Compound 55, and Compound 57 of the present invention are self-made, and their chemical names and preparation methods are shown in the preparation examples of each compound.
[0185] Test animals: male SD rats, 3 / administration dose / test product, body weight 210-290g / rat.
[0186] Preparation of the test solution:
[0187] Preparation of blank solvent:
[0188] Preparation of 28% Captisol solution: Weigh 8.4g of Captisol, add a small amount of purified water to dissolve it ultrasonically, then dilute to 30mL with purified water, vortex and mix to obtain the product.
[0189] Preparation of 28% and 40% HP-β-CD solutions: Weigh 2.8g and 4.0g of HP-β-CD, prepare 10mL solutions with sterilized water for injection, and vortex to obtain the solution.
[0190](1) Compound 2, intravenous injection (iv) prescription: 5% DMSO+10% PEG400+85...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com