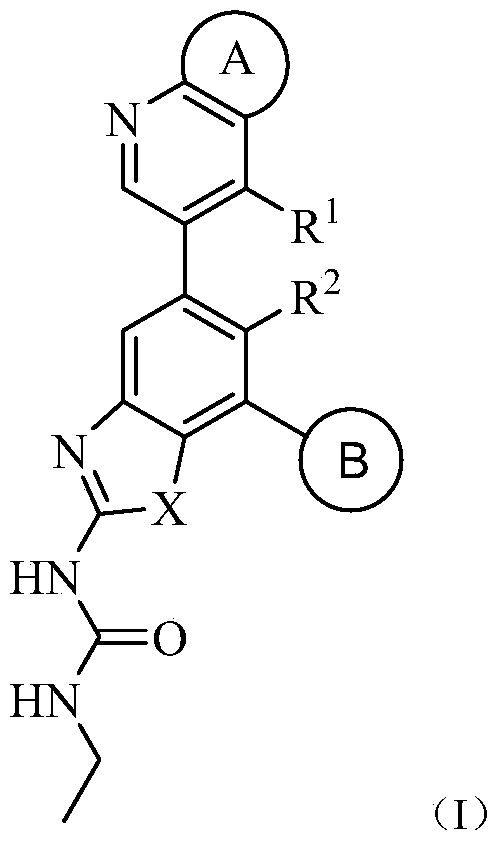
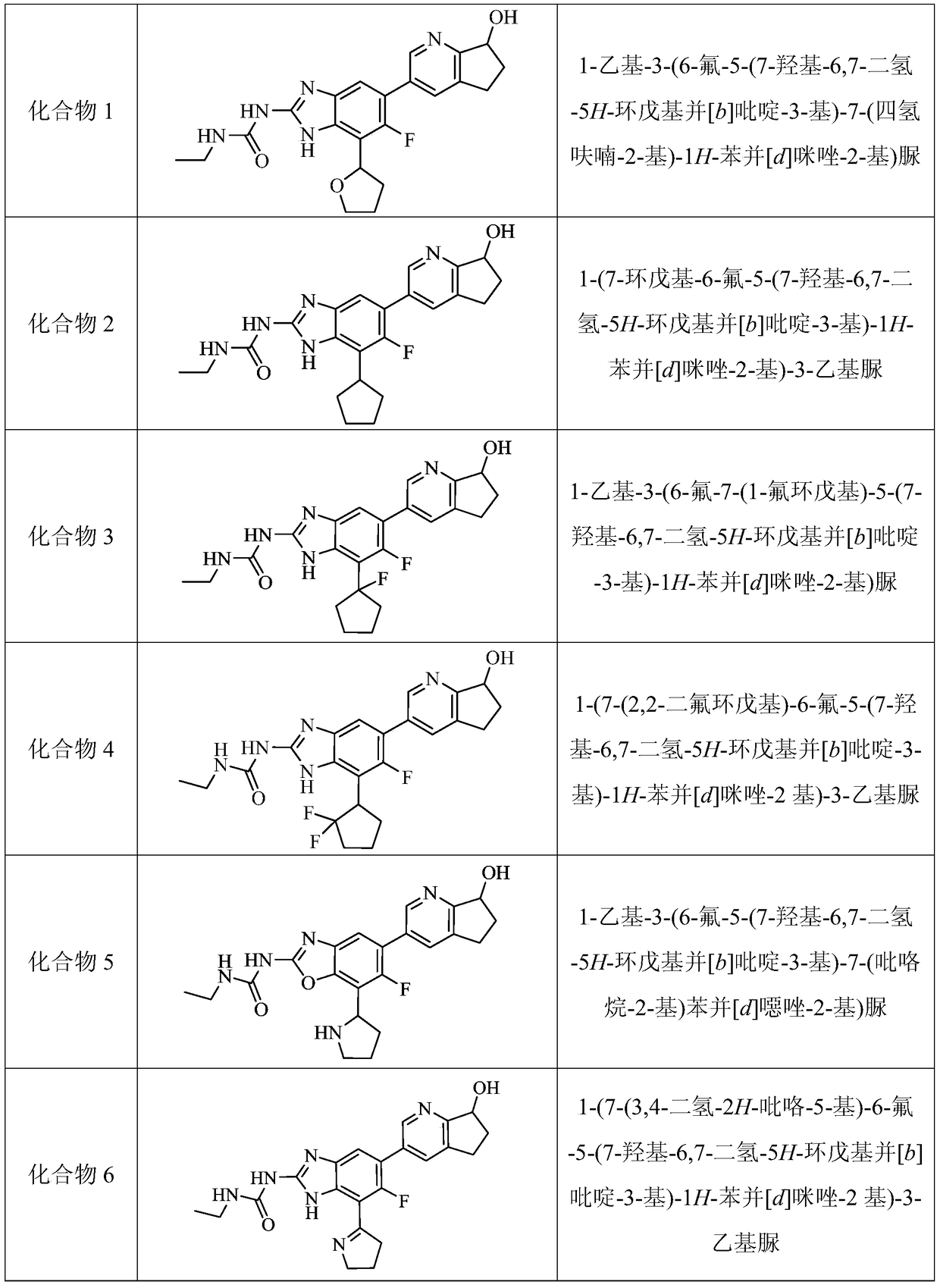
Pycyclic gyrase and topoisomerase iv inhibitors
A stereoisomer, heterocyclic group technology, applied in the direction of anti-inflammatory agents, non-central analgesics, medical preparations containing active ingredients, etc., can solve the problem of poor solubility, poor bacterial outer membrane penetration, and no clinical application and other issues
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0103] The present invention also provides the preparation method of above-mentioned compound:
[0104]
[0105] Reaction steps:
[0106] Step 1: Preparation of Intermediate 1
[0107] Dissolve raw material 1 and raw material 2 in an organic solvent (such as 1,4-dioxane, tetrahydrofuran), add potassium acetate, and add tricyclohexylphosphine and palladium catalyst (such as tris(dibenzylideneacetone) Dipalladium), heated to completion of the reaction. The reaction solution was directly used in the next reaction.
[0108] Step 2: Preparation of Intermediate 2
[0109] Add raw material 3, alkaline aqueous solution (such as sodium bicarbonate) and palladium catalyst (such as [1,1'-bis (diphenylphosphino) ferrocene] dichloropalladium dichloromethane complex ), heated under nitrogen until the end of the reaction. Cool, filter with suction, concentrate and purify to obtain intermediate 2.
[0110] Step 3: Preparation of intermediate 3
[0111] Add intermediate 2 into a mixe...
Embodiment 1
[0152] Example 1 1-Ethyl-3-(6-fluoro-5-(7-hydroxy-6,7-dihydro-5H-cyclopentyl[b]pyridin-3-yl)-7- Preparation of (tetrahydrofuran-2-yl)-1H-benzo[d]imidazol-2-yl)urea (Compound 1)
[0153]
[0154] 1) Preparation of 6,7-dihydro-5H-cyclopentyl[b]pyridine 1-oxide
[0155]
[0156] 6,7-dihydro-5H-cyclopentyl[b]pyridine (1.4g, 11.7mmol) was added to dichloromethane (40mL), and 3-chloroperoxybenzoic acid (mass fraction 77% , 5.25g, 23.4mmol), heated to reflux for 2 hours, down to room temperature, added calcium hydroxide (3.47g, 46.8mmol), stirred for 16 hours, suction filtered, the filter cake was washed with dichloromethane, and the organic phases were combined, Concentration in vacuo afforded the title compound (1.38 g, 87.3% yield).
[0157] 2) Preparation of 6,7-dihydro-5H-cyclopentyl[b]pyridin-7-yl acetate
[0158]
[0159] Add 6,7-dihydro-5H-cyclopenta[b]pyridine 1-oxide (1.38g, 10.2mmol) into acetic anhydride (30mL), heat up to 55°C for 18 hours, concentrate, ad...
Embodiment 2
[0177] Example 2 1-ethyl-3-(6-fluoro-5-(8-hydroxyl-5,6,7,8-tetrahydroquinolin-3-yl)-7-(tetrahydrofuran- Preparation of 2-yl)-1H-benzo[d]imidazol-2-yl)urea (compound 17)
[0178]
[0179] 1) Preparation of 3-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)-5,6,7,8-tetrahydroquinoline
[0180]
[0181] 5,6,7,8-tetrahydroquinoline (2.0g, 15mmol), bis(1,5-cyclooctadiene)di-μ-methoxydiiridium (I) (297.4mg, 0.45mmol) , 4,4'-di-tert-butyl-2,2'-bipyridine (241mg, 0.9mmol) and biboronic acid pinacol ester (3.81g, 15mmol) were added to tetrahydrofuran (40mL), under nitrogen protection at 75°C After reacting for 11 hours, the crude product (3.89 g) was obtained after concentration, which was directly carried out to the next reaction.
[0182] 2) Preparation of 3-bromo-5,6,7,8-tetrahydroquinoline
[0183]
[0184] 3-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)-5,6,7,8-tetrahydroquinoline crude product (3.89 g, 15mmol) was dissolved in methanol (80mL) and copper bromide (11.7...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com