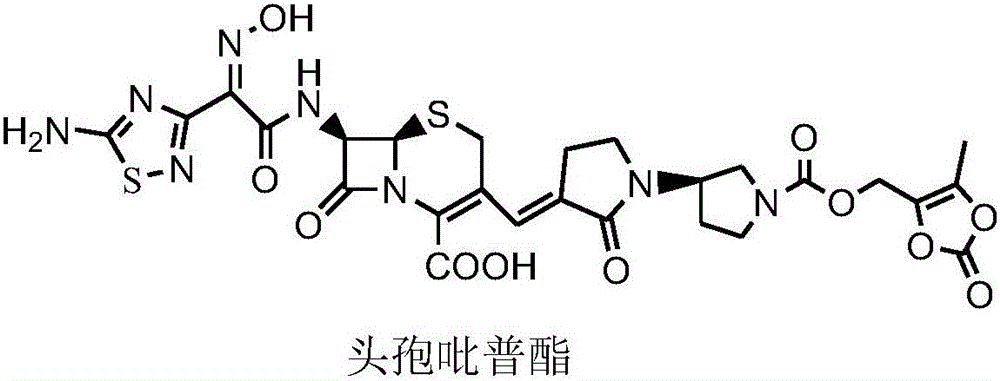
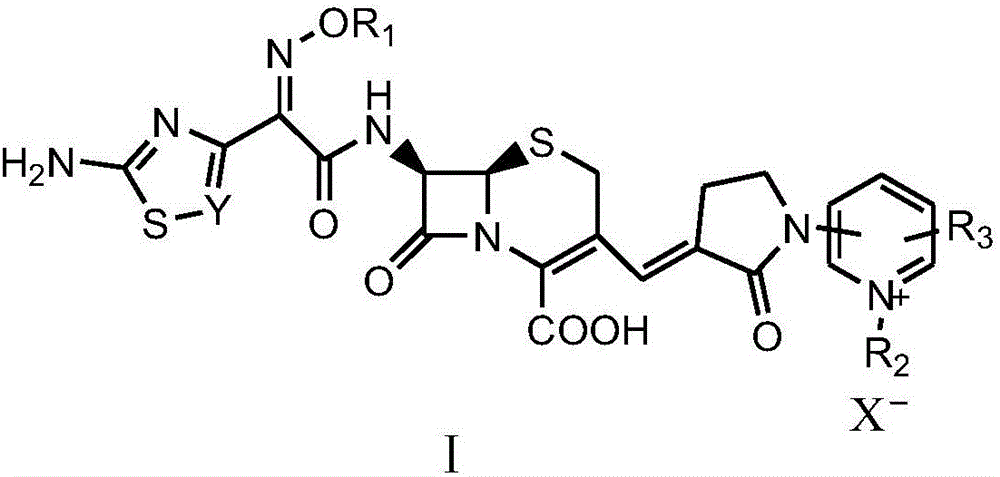
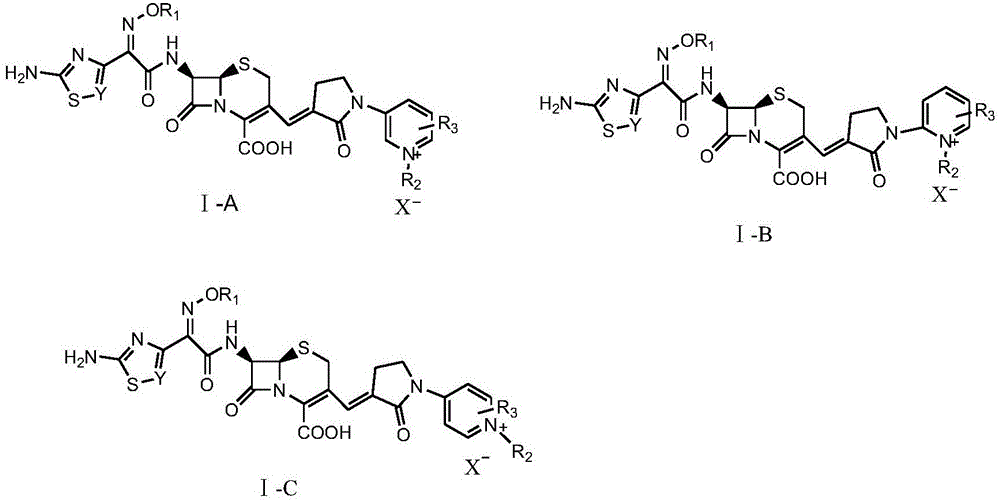
Pyridine-ion-containing vinyl cephalosporin derivative as well as preparation method and application thereof
An ion and alkenyl technology, applied in the field of medicine, can solve problems such as incomplete clinical data
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example 1
[0110] (6R,7R)-7-[(Z)-2-(5-amino-[1,2,4]thiadiazol-3-yl)-methoxyimino-acetylamino]-3-hydroxymethyl Preparation of Benzyl-8-oxo-5-thia-1-aza-bicyclo[4.2.0]oct-2-ene-2-carboxylate
[0111]
[0112] At 15℃, treat 11.5g (50mmol) (6R,7R)-7-amino-3-hydroxymethyl-8-oxo-5-sulfide with 6.3ml 1,1,3,3-tetramethyl biguanide Hetero-1-aza-bicyclo[4.2.0]oct-2-ene-2-carboxylic acid in 100ml N,N-dimethylformamide (DMF) suspension, stirring until it becomes a solution (5min) . Use 23.5 g (67 mmol) of (Z)-(5-amino-[1,2,4]thiadiazol-3-yl)-methoxyimino-thioacetic acid-S-benzene at 0°C After treatment with thiazol-2-yl ester, stirring was continued at 0°C for 5h, and the reaction was detected to be complete. The solution was diluted with 200 ml of water, and the aqueous layer was washed 3 times with 100 ml of ethyl acetate. The aquifer was cooled to 0°C, 400ml of dichloromethane solution containing 28g (144mmol) of diphenyldiazomethane was added, the pH value was adjusted to 3 with hydrochloric ac...
preparation example 2
[0114] (6R,7R)-7-[(Z)-2-(5-amino-[1,2,4]thiadiazol-3-yl)-ethoxyimino-acetylamino]-3-hydroxymethyl Preparation of benzhydryl-8-oxo-5-thia-1-aza-bicyclo[4.2.0]oct-2-ene-2-carboxylate.
[0115]
[0116] At 15℃, treat 11.5g (50mmol) (6R,7R)-7-amino-3-hydroxymethyl-8-oxo-5-sulfide with 6.3ml 1,1,3,3-tetramethyl biguanide Hetero-1-aza-bicyclo[4.2.0]oct-2-ene-2-carboxylic acid in 100ml N,N-dimethylformamide (DMF) suspension, stirring until it becomes a solution (5min) . Use 24.5 g (67 mmol) of (Z)-(5-amino-[1,2,4]thiadiazol-3-yl)-ethoxyimino-thioacetic acid-S-benzene at 0°C After treatment with thiazol-2-yl ester, stirring was continued at 0°C for 5h, and the reaction was detected to be complete. The solution was diluted with 200 ml of water, and the aqueous layer was washed 3 times with 100 ml of ethyl acetate. The aquifer was cooled to 0°C, 400ml of dichloromethane solution containing 28g (144mmol) of diphenyldiazomethane was added, the pH value was adjusted to 3 with hydrochloric...
preparation example 3
[0118] (6R,7R)-7-[(Z)-2-(5-amino-[1,2,4]thiadiazol-3-yl)-methoxyimino-acetylamino]-3-formyl Preparation of -8-oxo-5-thia-1-aza-bicyclo[4.2.0]oct-2-ene-2-carboxylate
[0119]
[0120] Add 5g (8.62mmol) of 7-[2-(5-amino-[1,2,4]thiadiazol-3-yl)-methoxyimino-acetylamino]-3-hydroxymethyl under 20℃ Benzyl-8-oxo-5-thia-1-aza-bicyclo[4.2.0]oct-2-ene-2-carboxylate, 0.5g TEMPO dissolved in 60ml dichloromethane, divided Add 30 g (345 mmol) of active manganese dioxide in 3 batches. After reacting at room temperature for 5 hours, it was detected that the raw materials were exhausted, filtered, and the filtrate was evaporated to dryness to obtain a red solid. The product was obtained by column chromatography with petroleum ether / ethyl acetate = 1:1, and the yield was 58.2%. TOF-MS[M+H+]579.6; 1H NMR(300MHz, CDCl 3 )δ=9.70(s,1H),8.30(d,1H),7.3-7.4(m,10H),7.07(s,1H,CHPh 2 ), 6.40 (s, 2H), 6.27 (dd, 1H), 5.16 (d, 1H), 4.11 (s, 3H), 4.00 (d, J = 18.7, 1H), 3.31 (d, J = 18.7, 1H ).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com