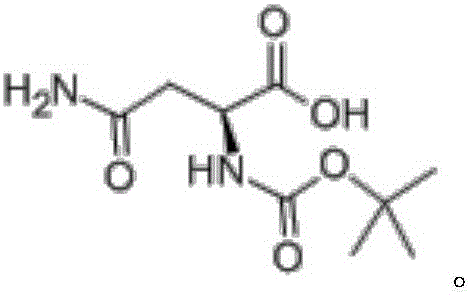
Preparation method of BOC-L-asparagine
A technology of asparagine and asparagine, which is applied in the field of medicinal chemistry, can solve the problems of unfavorable industrialization, cumbersome operation, cost, environmental protection, and high safety, and achieve the effect of being conducive to industrialized production, simple operation, and high safety factor
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
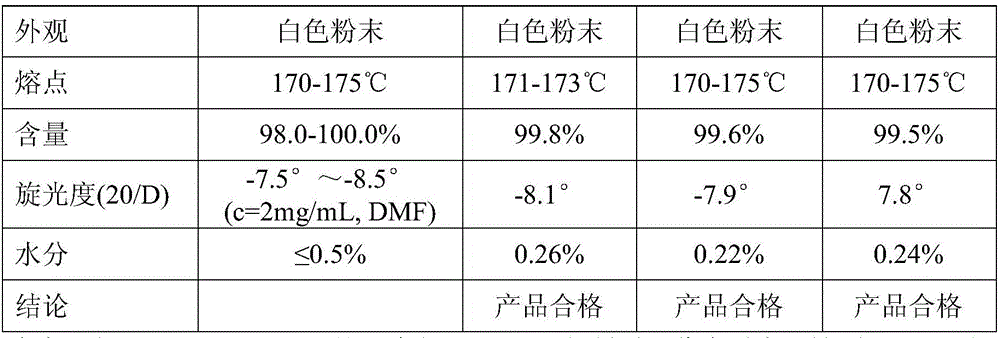
Embodiment 1
[0025] BOC-L-asparagine was prepared according to the following preparation steps:
[0026] Step (1), put 150g L-Asn (1mol), 240g di-tert-butyl dicarbonate (1.1mol) and 700mL water into the 3L flask; at a temperature of 25~30°C, under the condition that the rotation speed is maintained at 120 rpm , 300mL of 10mol / L NaOH aqueous solution (3mol) was added dropwise while stirring, and the rate of addition was controlled to make pH=9~10. During this process, L-Asn and di-tert-butyl dicarbonate were dissolved in water and reacted; the dropwise addition was completed. After that, the temperature was raised to 34°C, and the reaction was continued for 4 hours under stirring;
[0027] Step (2), the reaction solution is cooled to less than 10°C with ice brine; 6mol / L hydrochloric acid is slowly added dropwise, the temperature is kept lower than 10°C during the dropwise addition, acidified to pH4.3, and the BOC-L-asparagine crude product is separated out, Suction filtration, wash the so...
Embodiment 2
[0029] BOC-L-asparagine was prepared according to the following preparation steps:
[0030] Step (1), put 150g L-Asn (1mol), 262g di-tert-butyl dicarbonate (1.2mol) and 700mL water into the 3L flask; at a temperature of 25~30°C, under the condition that the rotation speed is maintained at 120 rpm , 300mL of 10mol / L NaOH aqueous solution (3mol) was added dropwise while stirring, and the rate of addition was controlled to make pH=9~10. During this process, L-Asn and di-tert-butyl dicarbonate were dissolved in water and reacted; the dropwise addition was completed. After that, the temperature was raised to 34°C, and the reaction was continued for 4 hours under stirring;
[0031] Step (2), the reaction solution is cooled to less than 10°C with ice brine, and residual (Boc) is detected 2 O crystals; slowly add 6mol / L hydrochloric acid dropwise, keep the temperature below 10 °C during the dropwise addition, acidify to pH 4.3, separate out the crude BOC-L-asparagine, filter with suc...
Embodiment 3
[0033] BOC-L-asparagine was prepared according to the following preparation steps:
[0034] 150g L-Asn (1mol), 218g di-tert-butyl dicarbonate (1mol) and 700mL water were put into the step (1), 3L flask; at a temperature of 25~30°C, under the condition that the rotation speed was maintained at 120 rev / min, 300mL of 10mol / L NaOH aqueous solution (3mol) was added dropwise while stirring, and the rate of addition was controlled to make pH=9~10. During this process, L-Asn and di-tert-butyl dicarbonate were dissolved in water and reacted; the dropwise addition was completed. After that, the temperature was raised to 34°C, and the reaction was continued for 4 hours under stirring;
[0035] Step (2), the reaction solution is cooled to less than 10°C with ice brine, and residual (Boc) is detected 2 O crystal; slowly add 6mol / L hydrochloric acid dropwise, keep the temperature below 10°C during the dropwise addition, acidify to pH 4.3, separate out the crude BOC-L-asparagine, filter wit...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com