Nanometer antibody Nb16 for anti-CTLA-4 and preparation method and application of nanometer antibody Nb16
A technology of nano-antibodies and recombinant vectors, which is applied in botany equipment and methods, biochemical equipment and methods, nanotechnology for materials and surface science, etc., and can solve the problems of long research and development cycle, difficulty in large-scale production, and easy contamination And other issues
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
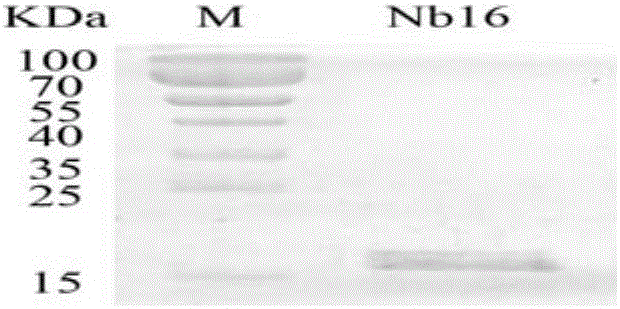
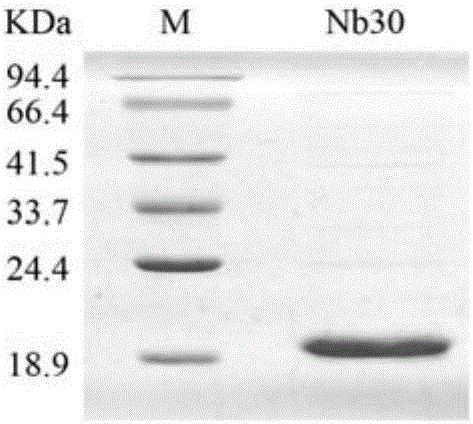
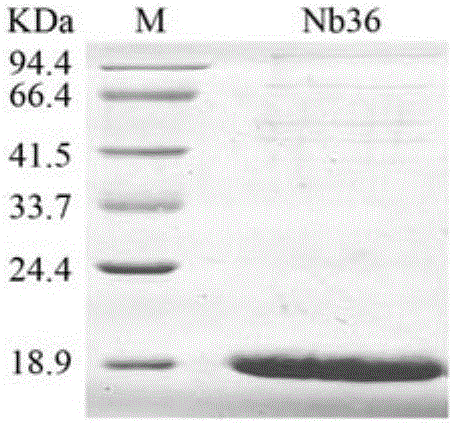
[0093] Embodiment 1, the preparation of nanobody
[0094] The present invention provides four nanobodies derived from camels, whose names are respectively Nb16, Nb30, Nb36 and Nb91. Wherein, the amino acid sequence of Nb16 is shown in SEQ ID No.1 in the sequence listing, encoded by the nucleotide sequence of SEQ ID No.2; the amino acid sequence of Nb30 is shown in SEQ ID No.3 in the sequence listing, encoded by SEQ ID No. The nucleotide sequence encoding of No.4; the amino acid sequence of Nb36 is as shown in SEQ ID No.5 in the sequence listing, encoded by the nucleotide sequence of SEQ ID No.6; the amino acid sequence of Nb91 is as shown in SEQ ID No in the sequence listing .7, encoded by the nucleotide sequence of SEQ ID No.8.
[0095] Replace the DNA fragment between the PstI and NotI recognition sequences of the vector pComb3 (Biovector product) with the DNA molecule shown in SEQID No.2, and keep the other sequences unchanged to obtain the recombinant vector pComb3-Nb16. ...
Embodiment 2
[0108] Example 2, Determination of Nanobody and CTLA-4 Binding Rate
[0109] 1. Determination of the binding rate of Nanobody to CTLA-4 (direct method)
[0110] T cells were isolated from peripheral blood cells of healthy volunteers and cultured in 96-well plates. After adding 10 μg / ml PHA (sigma, L9017) to T cells for co-culture for 72 hours, Nb16 (1 μg) from Example 1 was added to 1×10 6 Incubate the above T cells in the dark at 4°C for 30 min, wash with PBS three times, add 1 μg PE anti-HA tag antibody (abcam, Clone: 16B12) and incubate at 4°C for 30 min, wash with PBS three times, put the sample on the BACKMAN flow cytometer , the result is as Figure 5 Shown in A.
[0111] According to the above method, Nb16 was replaced by Nb30, Nb36 and Nb91 respectively, and other steps were kept unchanged, and the binding results of Nb30, Nb36 and Nb91 and CTLA-4 were obtained, respectively as follows Image 6 Middle A, Figure 7 Middle A, Figure 8 Shown in A.
[0112] 2. Det...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com