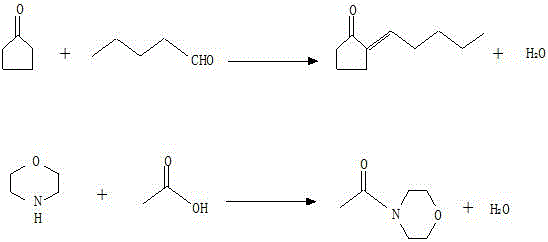
Method for synthesizing 2-pentylidene cyclopentanone using morpholine as catalyst
A technology of pentylidene cyclopentanone and cyclopentanone, which is applied in the field of synthesizing 2-pentylidene cyclopentanone with morpholine as a catalyst, can solve the problems of increased safety risks, complex process, increased energy consumption, etc., and achieve comprehensive cost Low, simple process flow, conducive to the effect of rectification and fractionation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
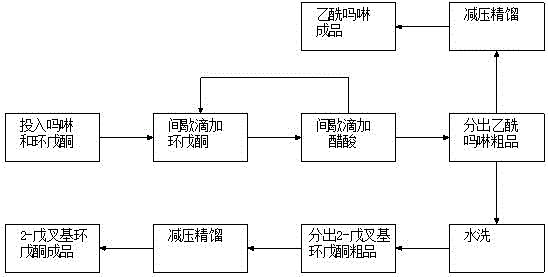
Image
Examples
Embodiment 1
[0018] Put 420g (5.00mol) of cyclopentanone and 84g (0.97mol) of morpholine into a 1-liter three-necked flask, raise the temperature to 80°C, add 215g of n-valeraldehyde (2.50mol) dropwise, add dropwise in 8 times, and add dropwise 1 / After 8 n-valeraldehyde, 1 / 8 glacial acetic acid was added dropwise, and 115 (1.92 mol) g of acetic acid was added dropwise 8 times for a total of 8 hours. The addition of n-valeraldehyde and acetic acid was completed, and the reaction was carried out at a constant temperature for 45 minutes. After standing still for 30 minutes, the crude product of acetylmorpholine in the lower layer was separated, washed with 127 g of water, and the aqueous phase in the lower layer was separated to obtain the crude product of 2-pentylidene cyclopentanone.
[0019] The crude acetylmorpholine was distilled under reduced pressure and deacidified, and the fraction at 94-97°C / 550Pa was collected to obtain 115.1g of acetylmorpholine finished product, with a GC conten...
Embodiment 2
[0022] Put 300g (3.57mol) of cyclopentanone and 90g (1.03mol) of morpholine into a 1-liter three-necked flask, raise the temperature to 60°C, add 307g (3.57mol) of n-valeraldehyde dropwise, add dropwise in 6 times, and add dropwise 1 / After 6 doses of n-valeraldehyde, 1 / 6 glacial acetic acid was added dropwise, the amount of acetic acid added was 62.1 g (1.03 mol) g in total, and the adding time was 6 hours. The addition of n-valeraldehyde and acetic acid was completed, and the reaction was carried out at a constant temperature for 60 minutes. After standing still for 30 minutes, the crude product of acetylmorpholine in the lower layer was separated. Add 121 g of water for washing, and remove the lower aqueous phase to obtain the crude product of 2-pentylidene cyclopentanone.
[0023] The crude acetylmorpholine was distilled under reduced pressure and deacidified, and the fraction at 94-97°C / 550Pa was collected to obtain 112.5g of acetylmorpholine finished product, with a GC ...
Embodiment 3
[0026] Put 704g (8.38mol) of cyclopentanone and 66g (0.76mol) of morpholine into a 2-liter three-necked flask, raise the temperature to 100°C, add 616g of n-valeraldehyde (7.16mol) dropwise, add dropwise in 10 times, and add dropwise 1 / After 10 n-valeraldehyde, add 1 / 10 glacial acetic acid dropwise, and add 68.3 (1.14 mol) g of acetic acid dropwise for 10 times, for a total of 10 hours. The addition of n-valeraldehyde and acetic acid was completed, and the reaction was carried out at a constant temperature for 30 minutes. After standing still for 30 minutes, the crude product of acetylmorpholine in the lower layer was separated, washed with 264 g of water, and the aqueous phase in the lower layer was separated to obtain the crude product of 2-pentylidene cyclopentanone.
[0027] The crude acetylmorpholine was distilled under reduced pressure and deacidified, and the fraction at 94-97°C / 550Pa was collected to obtain 88.7g of acetylmorpholine finished product, with a GC content...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com