Syntheses and application of water-soluble bis-Schiff base potassium salts
A Schiff base potassium salt, water-soluble technology, applied in the field of Schiff base corrosion inhibitors and synthesis, can solve the problems of inability to achieve high concentration, low solubility, low slow release effect, etc. The effect of good performance, simple synthesis and separation methods
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
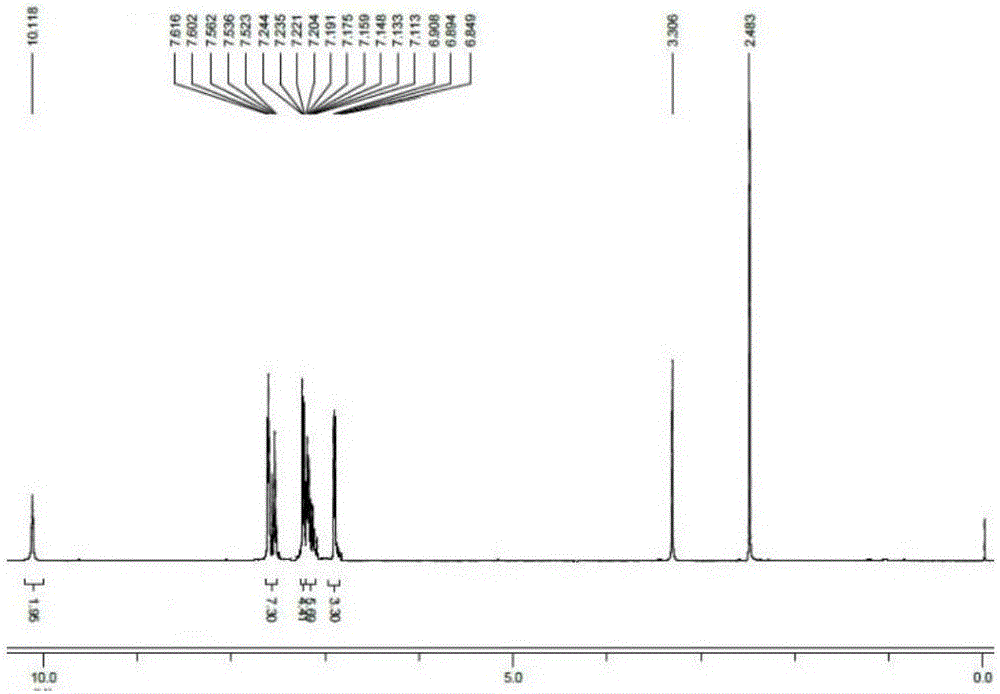
[0017] 2,2'-(1E,1'E)-(4,4'-((E)-ethylene-1,2-diyl)bis(4,1-phenylene))bis(azane-1 Synthesis of Potassium -yl-1-ylidene)di(methane-1-yl-1-ylidene)diphenylalkoxide
[0018] 2,2'-(1E,1'E)-(4,4'-((E)-ethylene-1,2-diyl)bis(4,1-phenylene))bis(azane-1 -yl-1-ylidene) bis(methane-1-yl-1-ylidene)diphenol (1.00g, 2.4mmol), potassium hydroxide (0.29g, 5.3mmol) were added to a 50mL single-necked flask, and 10mL of anhydrous Ethanol, stirred magnetically at normal temperature for 2 hours, the reaction was completed, and the solvent was removed by distillation under reduced pressure, then extracted with methylene chloride, and distilled under reduced pressure to obtain a yellow solid compound, and its hydrogen nuclear magnetic resonance spectrum was as follows: figure 1 , yield 65%. 1 H-NMR (400MHz, DMSO-d 6 )δ (ppm): 10.118 (s, 2H, -OH), 7.616-7.523 (m, 7H, Ar-H), 7.244-7.113 (m, 10H, Ar-H), 6.908-6.894 (d, J= 5.6Hz, 3H, Ar-H), 6.849-6.824 (t, 2H, J=5Hz, Ar-CH=CH).
Embodiment 2
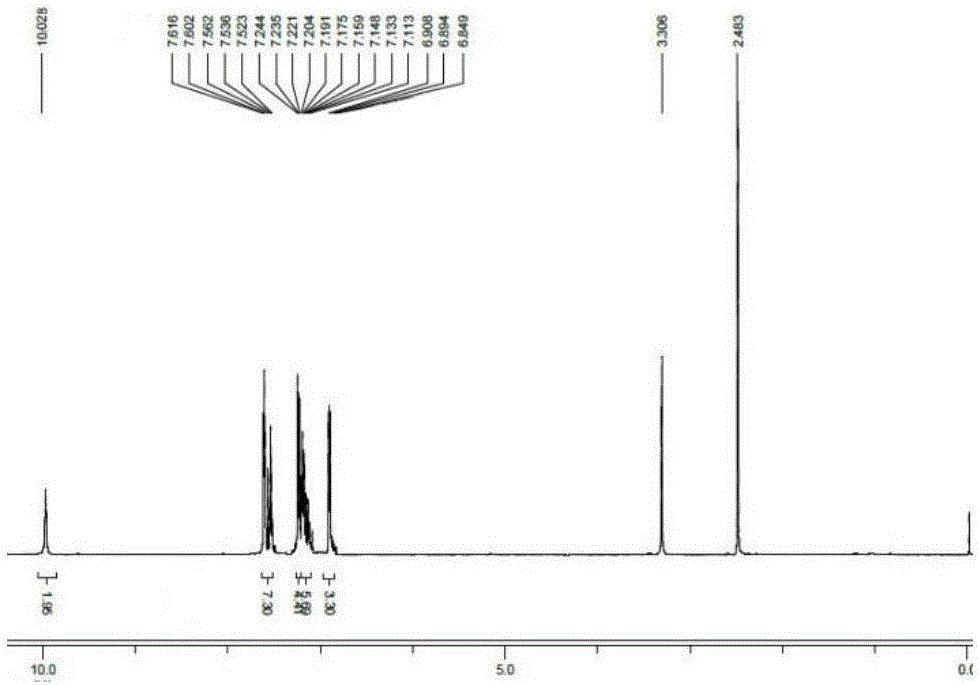
[0020] 3,3'-(1E,1'E)-(4,4'-((E)-ethylene-1,2-diyl)bis(4,1-phenylene))bis(azane-1 Synthesis of Potassium -yl-1-ylidene)di(methane-1-yl-1-ylidene)diphenylalkoxide
[0021] 3,3'-(1E,1'E)-(4,4'-((E)-ethylene-1,2-diyl)bis(4,1-phenylene))bis(azane-1 -yl-1-ylidene) bis(methane-1-yl-1-ylidene)diphenol (1.00g, 2.4mmol), potassium hydroxide (0.29g, 5.3mmol) were added to a 50mL single-necked flask, and 10mL of anhydrous Ethanol, magnetic stirring at normal temperature for 2 hours, the reaction was completed, the solvent was removed by distillation under reduced pressure, then extracted with dichloromethane, and distilled under reduced pressure to obtain a yellow solid compound, and its H NMR spectrum was as follows: figure 2 , yield 67%. 1 H-NMR (400MHz, DMSO-d 6 )δ (ppm): 10.028 (s, 2H, -OH), 7.616-7.523 (m, 7H, Ar-H), 7.244-7.113 (m, 10H, Ar-H), 6.908-6.894 (d, J= 5.6Hz, 3H, Ar-H), 6.849-6.824 (t, 2H, J=5Hz, Ar-CH=CH).
Embodiment 3
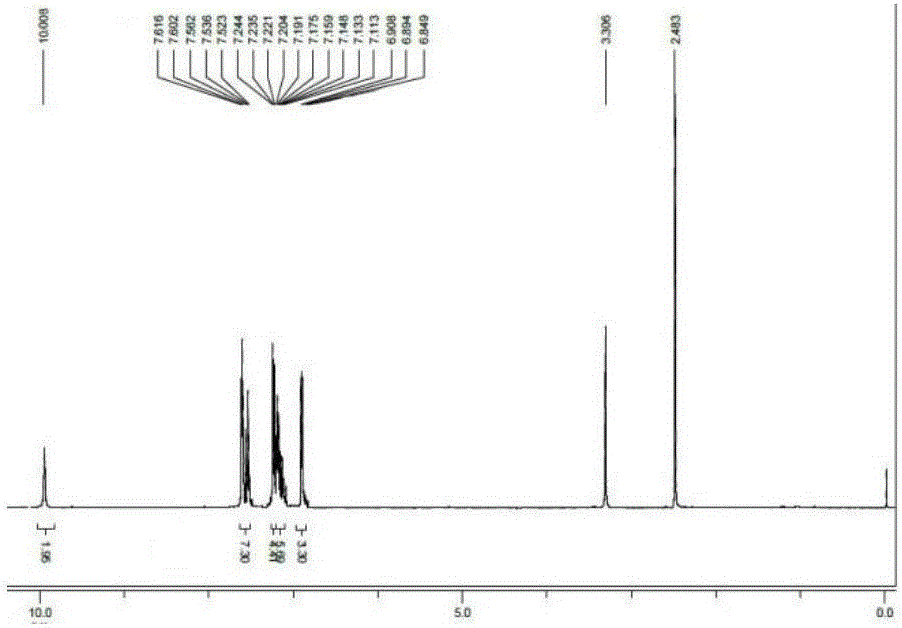
[0023] 4,4'-(1E,1'E)-(4,4'-((E)-ethylene-1,2-diyl)bis(4,1-phenylene))bis(azane-1 Synthesis of Potassium -yl-1-ylidene)di(methane-1-yl-1-ylidene)diphenylalkoxide
[0024] 4,4'-(1E,1'E)-(4,4'-((E)-ethylene-1,2-diyl)bis(4,1-phenylene))bis(azane-1 -yl-1-ylidene) bis(methane-1-yl-1-ylidene)diphenol (1.00g, 2.4mmol), potassium hydroxide (0.29g, 5.3mmol) were added to a 50mL single-necked flask, and 10mL of anhydrous Ethanol, stirred magnetically at normal temperature for 2 hours, the reaction was completed, and the solvent was removed by distillation under reduced pressure, then extracted with methylene chloride, and distilled under reduced pressure to obtain a yellow solid compound, and its hydrogen nuclear magnetic resonance spectrum was as follows: image 3 , yield 68%. 1 H-NMR (400MHz, DMSO-d 6 )δ (ppm): 10.008 (s, 2H, -OH), 7.616-7.523 (m, 7H, Ar-H), 7.244-7.113 (m, 10H, Ar-H), 6.908-6.894 (d, J= 5.6Hz, 3H, Ar-H), 6.849-6.824 (t, 2H, J=5Hz, Ar-CH=CH).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com