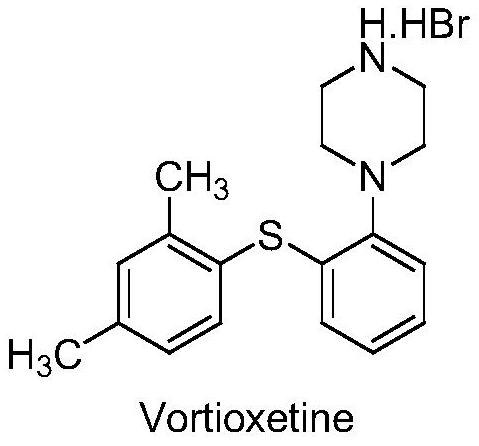
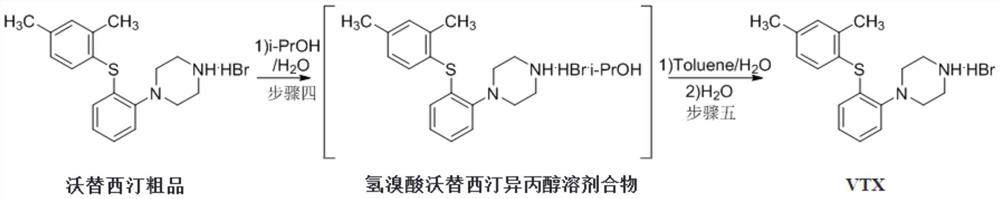
A kind of β-type efficient vortioxetine hydrobromide crystal conversion method
A technology of vortioxetine hydrobromide and hydrobromic acid, applied in organic chemistry methods, organic chemistry, etc., can solve the problems of complex synthesis and subsequent refining, and low total yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] Add 600 mL of mixed solvent IV to 100 g of tert-butyl-4-(2-(2,4-dimethylthiophenol) phenyl) piperazine-1-carboxylate and activated carbon (10 g) 异丙醇:40%氢溴酸 =10:1 (V:V), heated to react at 50°C for 1.5 hours, filtered while hot; the filter cake was pre-cooled with 50mL isopropanol and water mixed solvent (V 异丙醇:水 =10:1) rinse. Collect the filter cake, add 300 ml of water, distill out about 100 ml of solvent under normal pressure, stop heating, naturally cool to room temperature 15-25 ° C, stir and crystallize, filter, rinse the filter cake with water (50 mL), collect the filter cake, Vacuum drying at 50-55°C for 12-13 hours to obtain 71.0 grams of off-white crystalline solid powder, yield 75%, HPLC purity 99.96%, maximum single impurity no more than 0.1%, total impurity no more than 0.04%; residue on ignition <0.01 % qualified; GC detection of isopropanol was not detected; XRD was consistent with the vortioxetine hydrobromide β crystal form data reported by the original...
Embodiment 2
[0026] Add 800 mL of mixed solvent IV to 100 g of tert-butyl-4-(2-(2,4-dimethylthiophenol) phenyl) piperazine-1-carboxylate and activated carbon (10 g) 异丙醇:40%氢溴酸 =10:1 (V:V), heated to react at 50°C for 1.5 hours, filtered while hot; the filter cake was pre-cooled with 50mL isopropanol and water mixed solvent (V 异丙醇:水 =10:1) rinse. Collect the filter cake, add 300 ml of water, distill out about 100 ml of solvent under normal pressure, stop heating, naturally cool to room temperature 15-25 ° C, stir and crystallize, filter, rinse the filter cake with water (50 mL), collect the filter cake, Vacuum drying at 50-55°C for 12-13 hours to obtain 68.0 grams of off-white crystalline solid powder, yield 72%, HPLC purity 99.98%, maximum single impurity no more than 0.1%, total impurity 0.02%; residue on ignition <0.01% Qualified; isopropanol was not detected by GC; XRD was consistent with the β-crystal data of vortioxetine hydrobromide reported by the original CN102617513A.
Embodiment 3
[0028] Add 400 mL of mixed solvent IV to 100 g of tert-butyl-4-(2-(2,4-dimethylthiophenol) phenyl) piperazine-1-carboxylate and activated carbon (10 g) 异丙醇:40%氢溴酸 =10:1 (V:V), heated to react at 50°C for 1.5 hours, filtered while hot; the filter cake was pre-cooled with 50mL isopropanol and water mixed solvent (V 异丙醇:水 =10:1) rinse. Collect the filter cake, add 300 ml of water, distill out about 100 ml of solvent under normal pressure, stop heating, naturally cool to room temperature 15-25 ° C, stir and crystallize, filter, rinse the filter cake with water (50 mL), collect the filter cake, Vacuum drying at 50-55°C for 12-13 hours to obtain 75.0 grams of off-white crystalline solid powder, yield 79%, HPLC purity 99.88%, maximum simple impurity 0.07%, total impurity 0.12%; residue on ignition <0.01% qualified ; Isopropanol was not detected by GC; XRD was consistent with the vortioxetine hydrobromide β crystal form data reported by the original CN102617513A.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com