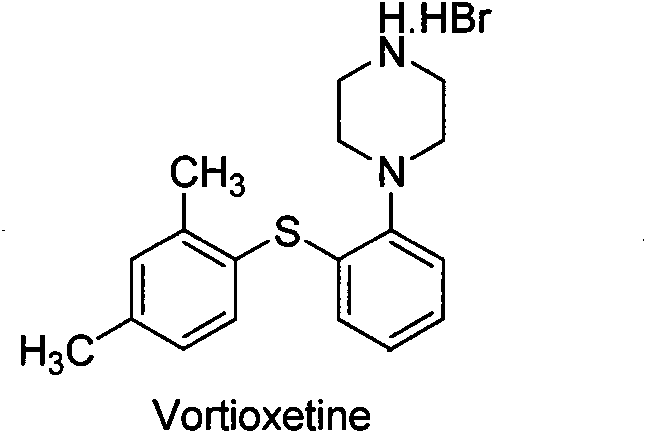
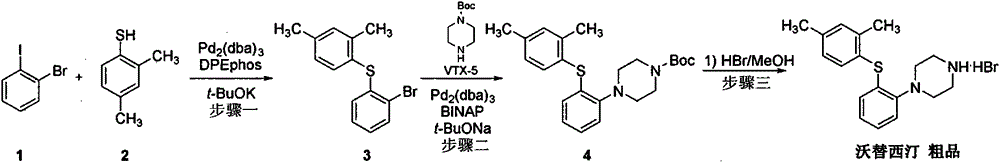
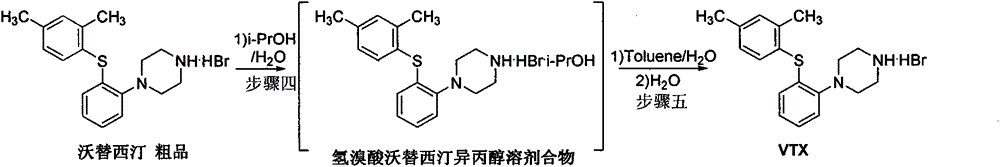
Beta type efficient vortioxetine hydrobromide crystal transformation method
A technology of vortioxetine hydrobromide and hydrobromic acid, applied in organic chemistry methods, organic chemistry, etc., can solve the problems of low total yield, cumbersome synthesis and subsequent refining, and achieve simplified refining operations and improved overall synthesis. Yield effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] In 100g tert-butyl-4-(2-(2,4-dimethylthiophenol)phenyl)piperazine-1-carbonate, activated carbon (10g) was added 600mL mixed solvent IV 异丙醇∶40%氢溴酸 =10:1 (V:V), heated and reacted at 50 ° C for 1.5 hours, filtered while hot; filter cake was mixed with pre-cooled 50mL isopropanol, water mixed solvent (V 异丙醇∶水 =10:1) Rinse. Collect the filter cake, add 300 ml of water, and distill about 100 ml of solvent at atmospheric pressure, stop heating, naturally cool to room temperature 15-25 ° C, stir for crystallization, filter, rinse the filter cake with water (50 mL), collect the filter cake, Vacuum drying at 50-55°C for 12-13 hours to obtain 71.0 g of off-white crystalline solid powder, yield 75%, HPLC purity 99.96%, maximum single impurity less than 0.1%, total impurity less than 0.04%; residue on ignition < 0.01 % qualified; isopropanol was not detected by GC; XRD was consistent with the β-crystal data of vortioxetine hydrobromide reported by the original research CN102617513...
Embodiment 2
[0027] In 100g tert-butyl-4-(2-(2,4-dimethylthiophenol)phenyl)piperazine-1-carbonate, activated carbon (10g) was added 800mL mixed solvent IV 异丙醇∶40%氢溴酸 =10:1 (V:V), heated and reacted at 50 ° C for 1.5 hours, filtered while hot; filter cake was mixed with pre-cooled 50mL isopropanol, water mixed solvent (V 异丙醇∶水 =10:1) Rinse. Collect the filter cake, add 300 ml of water, and distill about 100 ml of solvent at atmospheric pressure, stop heating, naturally cool to room temperature 15-25 ° C, stir for crystallization, filter, rinse the filter cake with water (50 mL), collect the filter cake, Vacuum drying at 50-55°C for 12-13 hours to obtain 68.0 g of off-white crystalline solid powder, yield 72%, HPLC purity 99.98%, maximum single impurity 0.1%, total impurity 0.02%; residue on ignition <0.01% Qualified; Isopropanol was not detected by GC; XRD was consistent with the β-crystal data of vortioxetine hydrobromide reported by the original research CN102617513A.
Embodiment 3
[0029] In 100g of tert-butyl-4-(2-(2,4-dimethylthiophenol)phenyl)piperazine-1-carbonate, activated carbon (10g) was added 400mL of mixed solvent IV 异丙醇∶40%氢溴酸 =10:1 (V:V), heated and reacted at 50 ° C for 1.5 hours, filtered while hot; filter cake was mixed with pre-cooled 50mL isopropanol, water mixed solvent (V 异丙醇∶水 =10:1) Rinse. Collect the filter cake, add 300 ml of water, and distill about 100 ml of solvent at atmospheric pressure, stop heating, naturally cool to room temperature 15-25 ° C, stir for crystallization, filter, rinse the filter cake with water (50 mL), collect the filter cake, Vacuum drying at 50-55°C for 12-13 hours to obtain 75.0 g of off-white crystalline solid powder, yield 79%, HPLC purity 99.88%, maximum single impurity 0.07%, total impurity 0.12%; residue on ignition < 0.01% qualified ; Isopropanol was not detected by GC detection; XRD was consistent with the β-crystal data of vortioxetine hydrobromide reported by the original research CN102617513A. ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com