Acenaphthene type polyphenyl copolymerized allyl phenolic aldehyde active dilute resin and preparation method thereof
A polyphenyl-copolymerized allyl phenolic and reactive dilution technology, applied in the field of resin and its preparation, can solve the problems of long reaction time, complicated modification process, cost reduction and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
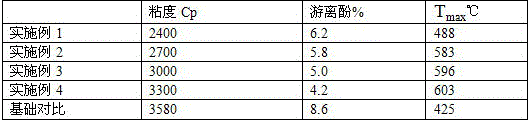
[0019] Add 100g of phenol solution and sodium hydroxide solution into the reaction kettle at about 65°C in sequence, then add 65g of paraformaldehyde into the reaction kettle in 3 times within half an hour, stir at high speed to mix evenly, react for 30min, and take 5 Slowly raise the temperature to 90°C at the rate of ℃ / min, stir at constant temperature for 1.5h, then cool to room temperature, neutralize the resin liquid with acetic acid, and then evaporate the small molecules in the system under reduced pressure until no liquid is discharged, and the viscous liquid is obtained as Phenolic Resin.
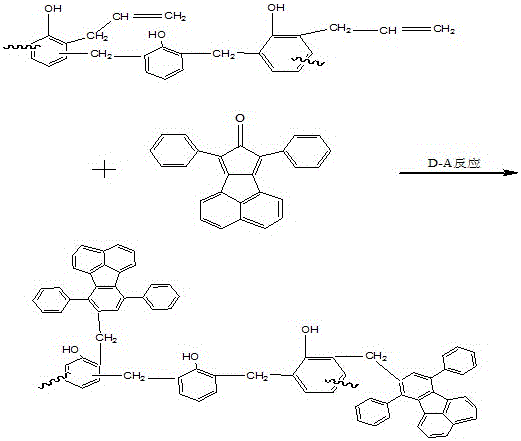
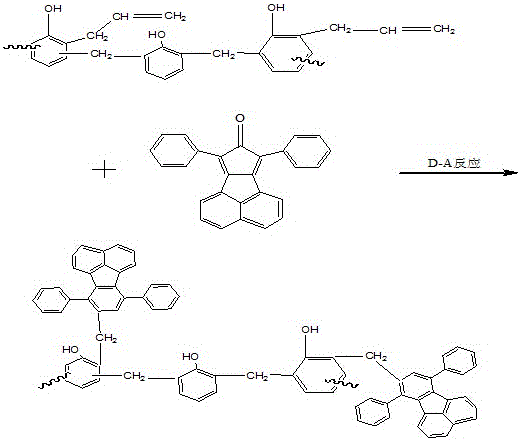
[0020] The synthesized phenolic resin is reacted with allyl chloride to generate allyl ether phenolic resin, and the resin undergoes Claisen rearrangement reaction at high temperature to generate allyl phenolic resin. At the same temperature, add 3g of acenaphthene-type cyclopentadienone and allyl resin to undergo Diels-Alder reaction, and finally obtain acenaphthene-type polypheny...
Embodiment 2
[0022] Add 100g of phenol solution and sodium hydroxide solution into the reaction kettle at about 65°C in sequence, then add 65g of paraformaldehyde into the reaction kettle in 3 times within half an hour, stir at high speed to mix evenly, react for 30min, and take 5 Slowly raise the temperature to 90°C at the rate of ℃ / min, stir at constant temperature for 1.5h, then cool to room temperature, neutralize the resin liquid with acetic acid, and then evaporate the small molecules in the system under reduced pressure until no liquid is discharged, and the viscous liquid is obtained as Phenolic Resin.
[0023] The synthesized phenolic resin is reacted with allyl chloride to generate allyl ether phenolic resin, and the resin undergoes Claisen rearrangement reaction at high temperature to generate allyl phenolic resin. At the same temperature, add 6g of acenaphthene-type cyclopentadienone and allyl resin to undergo Diels-Alder reaction, and finally obtain acenaphthene-type polypheny...
Embodiment 3
[0025] Add 100g of phenol solution and sodium hydroxide solution into the reaction kettle at about 65°C in sequence, then add 65g of paraformaldehyde into the reaction kettle in 3 times within half an hour, stir at high speed to mix evenly, react for 30min, and take 5 Slowly raise the temperature to 90°C at the rate of ℃ / min, stir at constant temperature for 1.5h, then cool to room temperature, neutralize the resin liquid with acetic acid, and then evaporate the small molecules in the system under reduced pressure until no liquid is discharged, and the viscous liquid is obtained as Phenolic Resin.
[0026] The synthesized phenolic resin is reacted with allyl chloride to generate allyl ether phenolic resin, and the resin undergoes Claisen rearrangement reaction at high temperature to generate allyl phenolic resin. At the same temperature, add 9g of acenaphthene-type cyclopentadienone and allyl resin to undergo Diels-Alder reaction, and finally obtain acenaphthene-type polypheny...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com