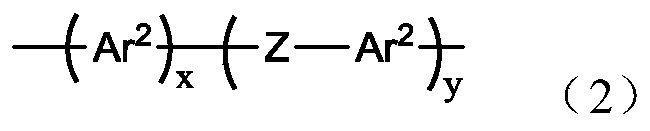Patents
Literature
34 results about "Cyclopentadienone" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Cyclopentadienone is an organic compound with molecular formula C₅H₄O. The parent cyclopentadienone is rarely encountered, because it rapidly dimerizes. Many substituted derivatives are known, notably tetraphenylcyclopentadienone. Such compounds are used as ligands in organometallic chemistry.
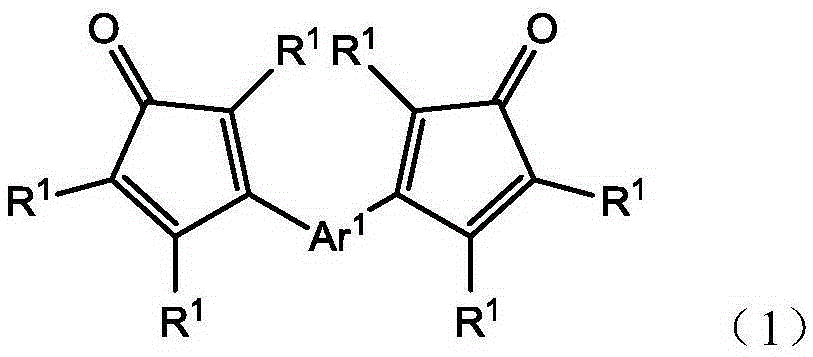
Polyphenylene oligomers and polymers
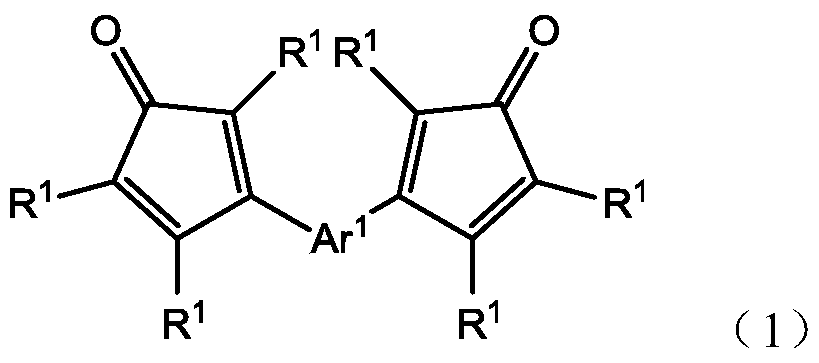
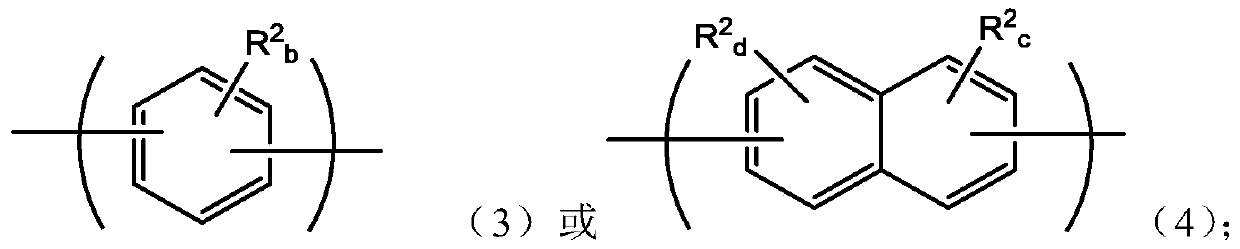
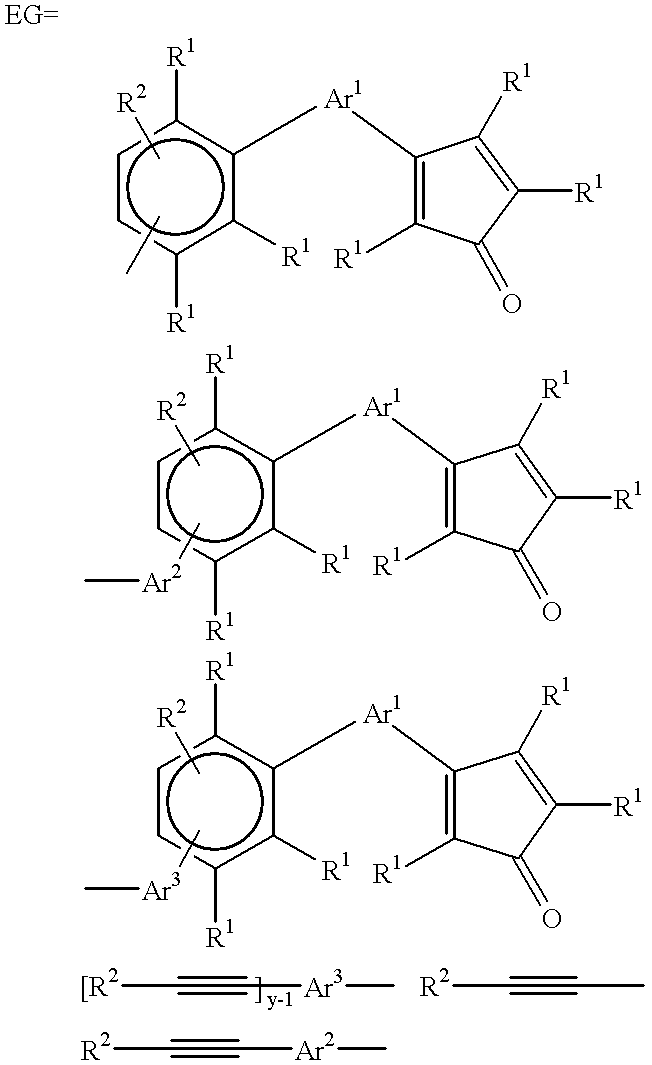
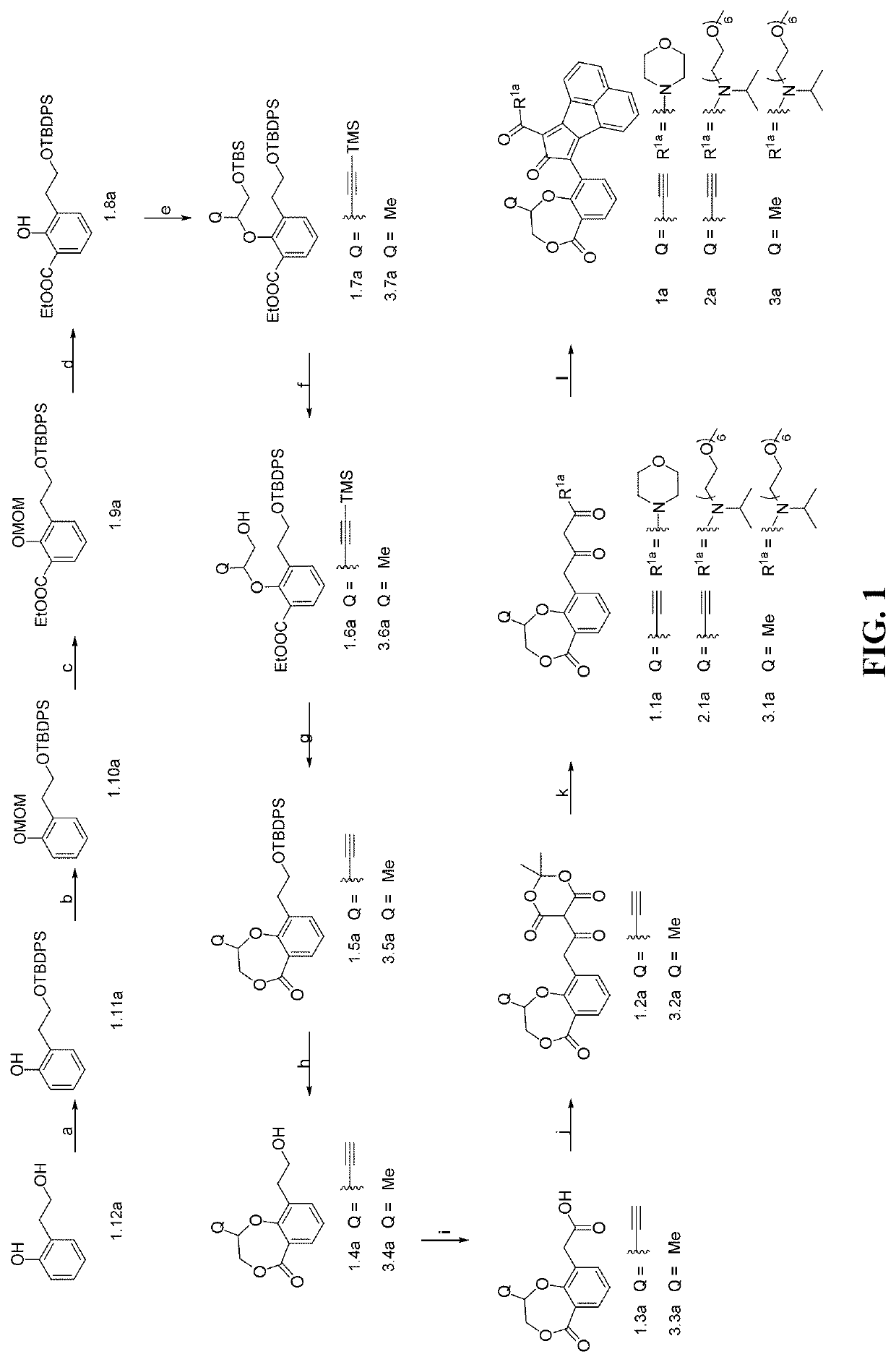
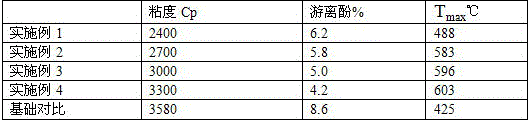
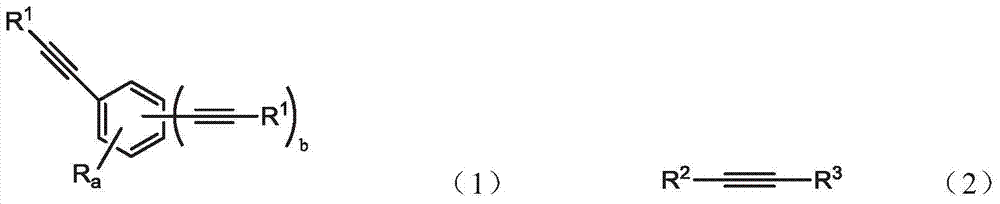
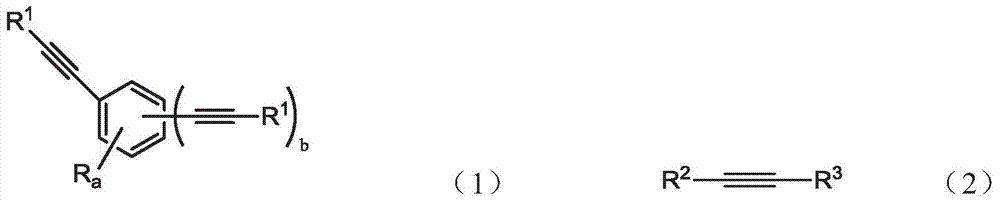
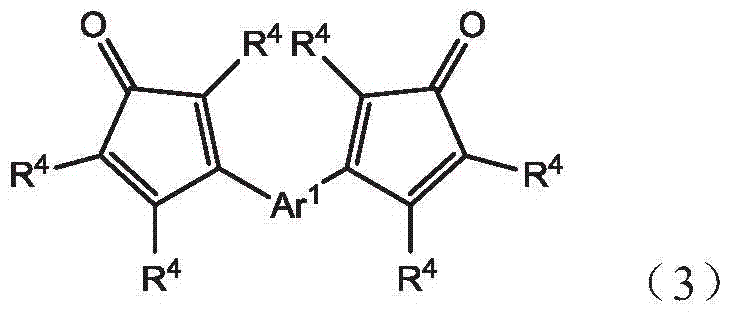
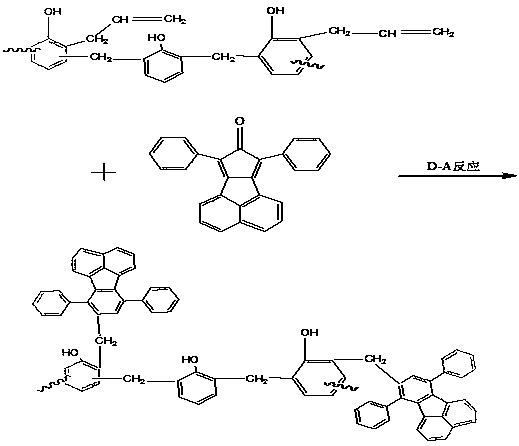
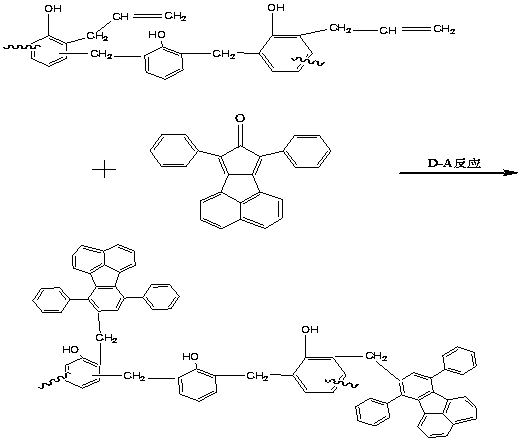
An oligomer, uncured polymer or cured polymer comprising the reaction product of one or more polyfunctional compounds containing two or more cyclopentadienone groups and at least one polyfunctional compound containing two or more aromatic acetylene groups wherein at least some of the polyfunctional compounds contain three or more reactive groups. Such oligomers and uncured polymers may be cured to form cured polymers which are useful as dielectrics in the microelectronics industry, especially for dielectrics in integrated circuits.
Owner:THE DOW CHEM CO
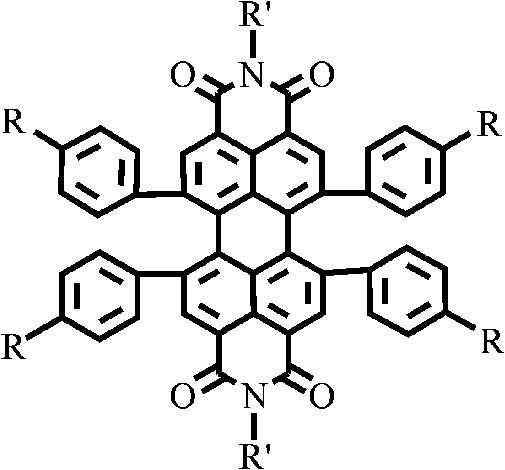
1, 6, 7, 12-tetraphenyl perylene bisimide derivant and preparation method thereof
InactiveCN101665492AImprove solubilityGood light and heat stabilityOrganic chemistryAnthracene dyesSolubilityKetone
The invention relates to a 1, 6, 7, 12-tetraphenyl perylene bisimide derivant and a preparation method thereof, belonging to the field of organic photoelectronic functional material preparation. The structural characteristic of the perylene bisimide derivant is that 1, 6, 7, 12-four harbor positions of 3, 4, 9, 10-perylene bisimide are connected with charge transport functional groups with different structure types and different conjugation degrees by phenyls. The derivant is synthesized by the following steps: firstly, a perylene bisimide core with terminal ethyne or halogen or other active groups at the periphery is synthesized by 1, 6, 7, 12-tetraphenyl perylene; and then the 1, 6, 7, 12-tetraphenyl perylene bisimide and cyclopentadienyl ketone with functional substitute group at periphery or arylamine and the like generate Diels-Alder cycloaddtion or C-N, C-C coupling reaction and the like, thus obtaining the target derivant. The compound has excellent thermal stability and morphologic stability and dissolvability; the opto-electronic property of the compound has obvious bathochromic shift compared with other perylene bisimide derivants; and the derivant can be widely applied in the fields of photoelectron and biology such as organic electroluminescence, solar cell, near-infrared fluorescence probe and the like.
Owner:DALIAN UNIV OF TECH
Synthesis method of indenofluorene derivatives, isotruxene and mono-substituted isotruxene derivatives
InactiveCN102627522AOrganic compound preparationHydrocarbon from oxygen organic compoundsEthyl groupKetone
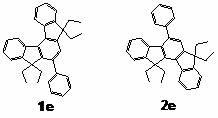
The invention discloses a synthesis method of indenofluorene derivatives, isotruxene and mono-substituted isotruxene derivatives. The synthesis method of indenofluorene derivatives is characterized in that ester-group-containing compounds are formed through Diels-Alder reaction of methyl propiolate and indenocyclopentadienone, ethyl substituted indenofluorene derivatives are formed through hydrolysis, acid catalytic ring closing, carbonyl reduction and introduction of ethyl onto methylene, diketone compounds produced after ring closing react with lithium salt of 4, 4'-di-tert-butyl-2-brominated biphenyl, and then acidification and ring closing are conducted to obtain indenofluorene derivatives with spirofluorene. The synthesis method of isotruxene is characterized in that 1, 4-diphenyl-2, 3-di (carbalkoxy) fluorenone products are formed through the Diels-Alder reaction of indenocyclopentadienone and dimethyl acetylenedicarboxylate, isotruxene ketone is obtained through hydrolysis and acid catalytic ring closing and finally isotruxene is obtained; dibenzyl alcohol products are formed through the Diels-Alder reaction of 1, 4-butynediol and indenocyclopentadienone, and isotruxene is obtained through acetone protection, carbonyl reduction, acetone removal and polyphosphoric acid (PPA) ring closing; and oriented oxy substituted products, i.e. isotruxene ketone which is derived from isotruxene with methylene at a No.5 position being substituted, and corresponding diethyl substituted products are additionally obtained. The indenofluorene derivatives, the isotruxene and the mono-substituted isotruxene derivatives disclosed by the invention can be used in the field of organic electroluminescence and organic micro-molecule solar cells.
Owner:EAST CHINA NORMAL UNIV
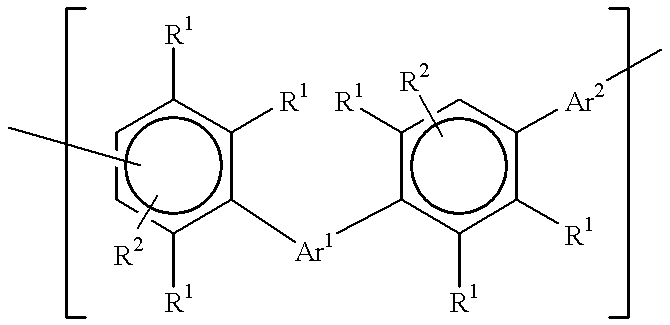
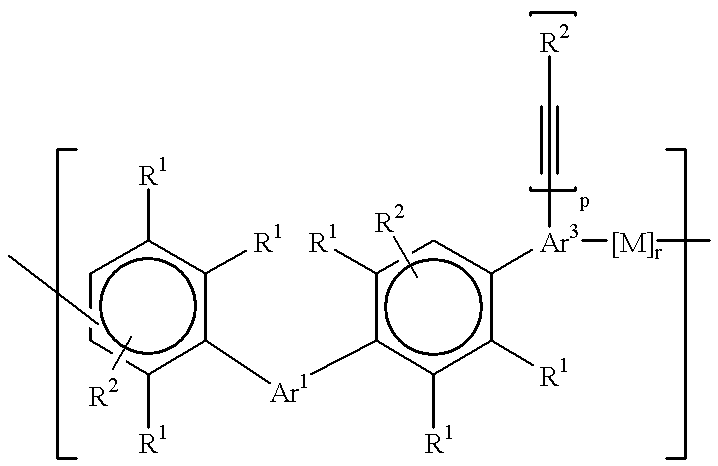
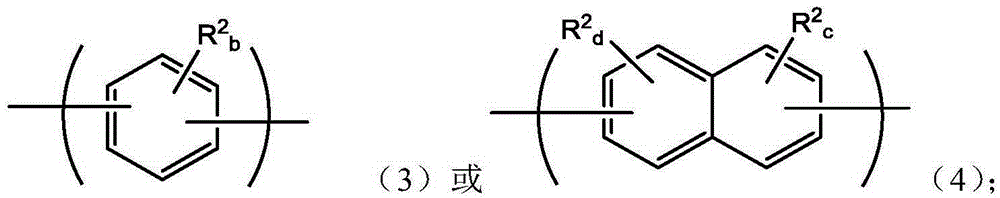
Polyarylene materials
ActiveCN105384915ASemiconductor/solid-state device detailsSolid-state devicesOligomerCyclopentadienone
Certain polyarylene oligomers having improved solubility are useful in forming dielectric material layers in electronics applications.
Owner:ROHM & HAAS ELECTRONICS MATERIALS LLC
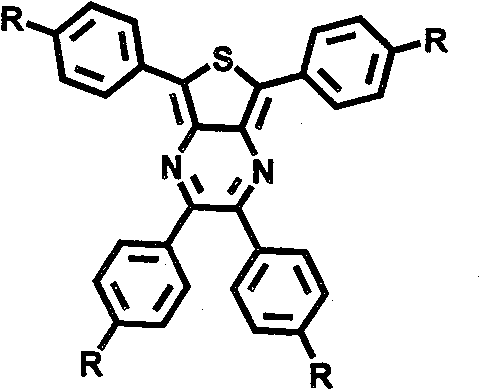
Thiophene and pyrazine derivatives and preparation method thereof
InactiveCN101830910AImprove solubilityGood light and heat stabilityOrganic chemistryLuminescent compositionsSynthesis methodsFluorescence
The invention relates to thiophene and pyrazine derivatives and a preparation method thereof, belonging to the field of organic electroluminescent materials. The derivatives are prepared by forming a molecular luminous core by centrosymmetrically connecting thiophene and pyrazine with four benzene rings, connecting functional groups of different structures and different conjugated degrees with the periphery of the core so that the red fluorescent molecules have charge-transmitting capability and can be used as a main red luminous material in the field of organic electroluminescence. The synthesis method comprises the following steps of: using 2,5-dibromothiophene as a raw material; nitrifying, carrying out C-C coupling with bromobenzene boric acid; reducing nitro groups; synthesizing a thiophene and pyrazine core with four peripheral bromines periphery together with 4,4'-dibromo benzyl; and then, carrying out C-N, C-C coupling reactions with arylamine, and the like or introducing acetylene groups to the periphery of the core and carrying out Diels-Alder cycloaddition with cyclopentadiene ketene with functional substituted groups to prepare target derivatives. The derivatives have strong absorption capability in an ultraviolet-visible light region, the dilute solution thereof emits strong fluorescence, and luminous peaks are between 600nm and 650nm.
Owner:DALIAN UNIV OF TECH
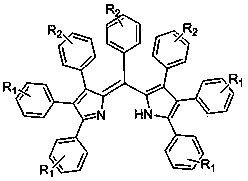
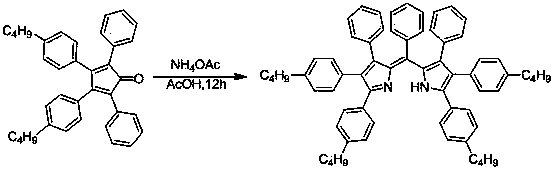
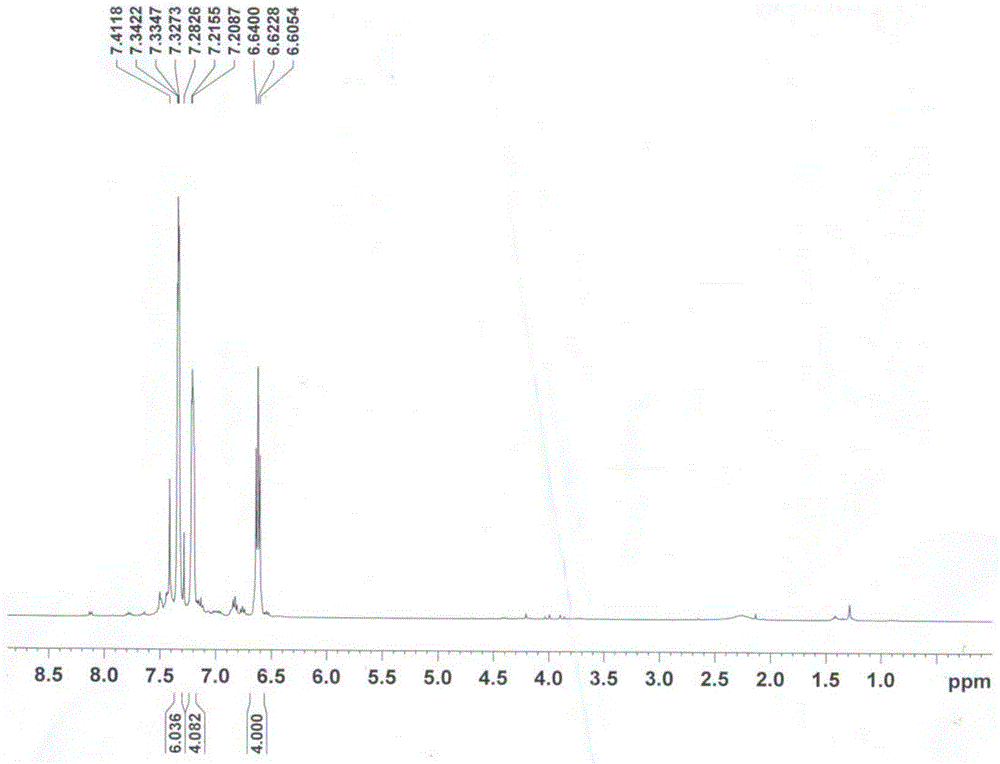
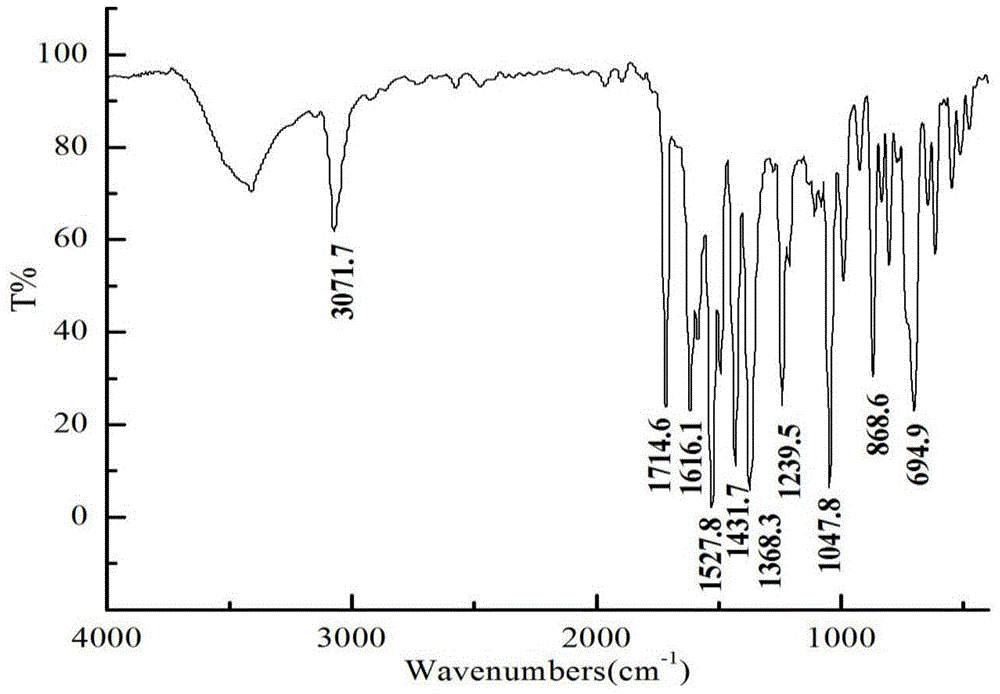
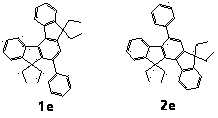
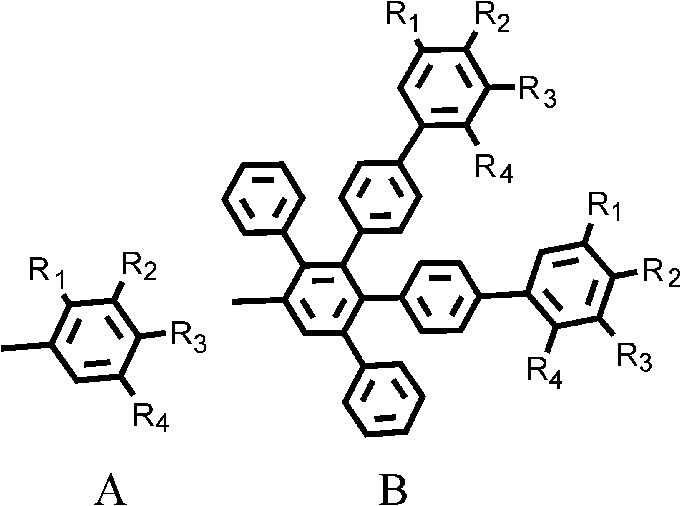
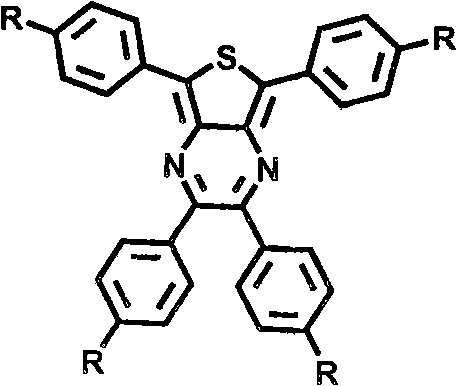
A 2,3,5,6,7,8-hexa-substituted imidazo[1,2-a]pyridine fluorescent material and its synthesis method
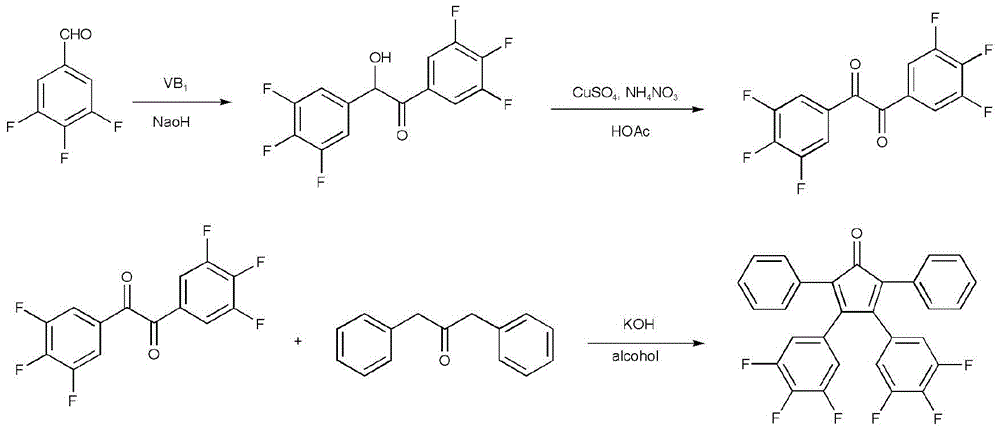
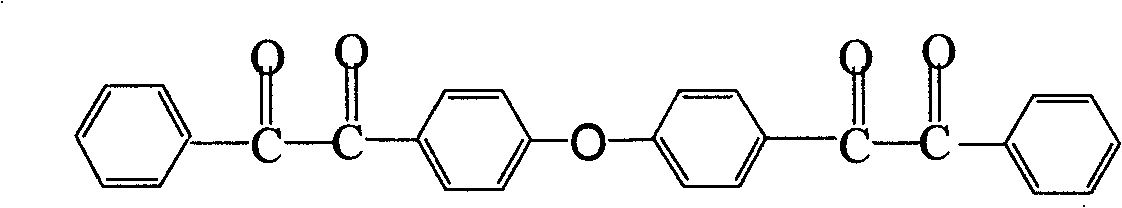
InactiveCN102286278ARaw materials are easy to getEasy to operateOrganic chemistryLuminescent compositionsFluorescenceSynthesis methods
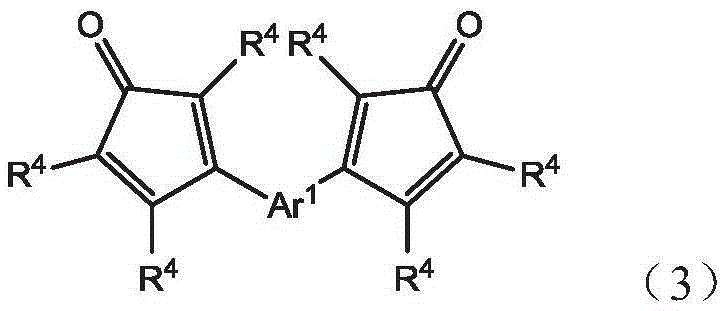
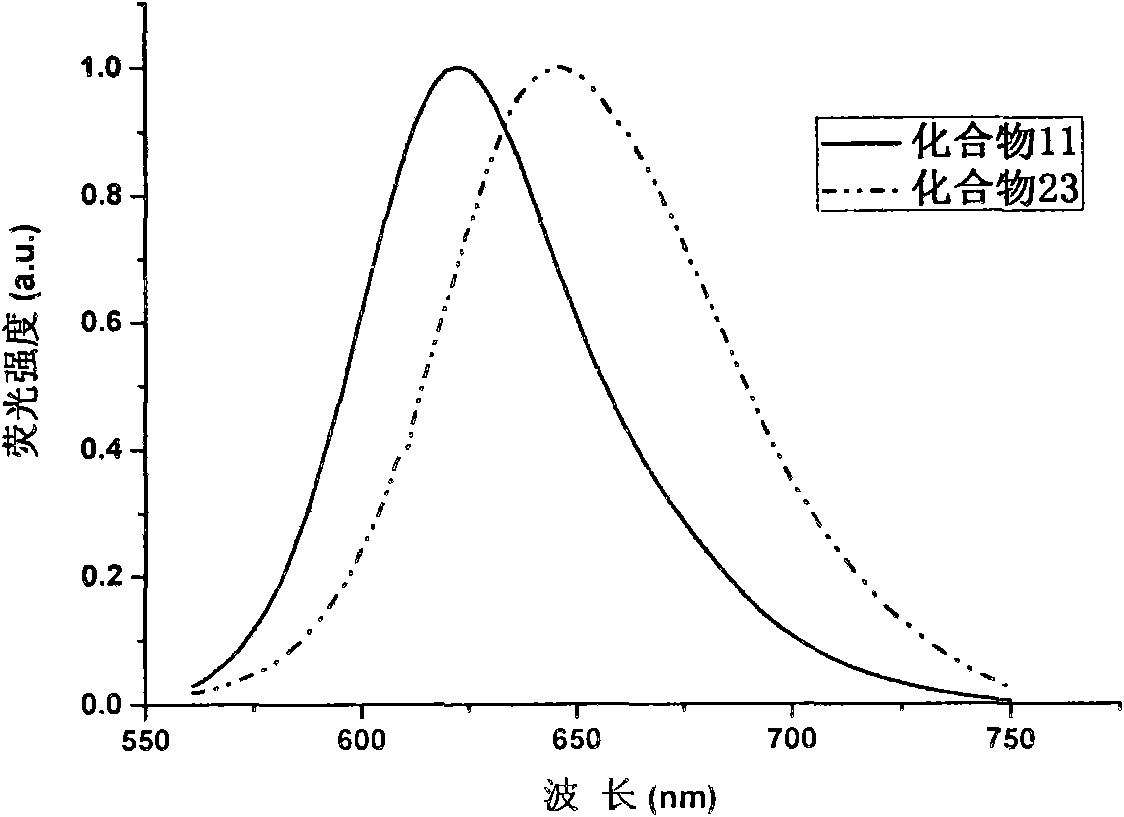
The invention discloses a 2,3,5,6,7,8-hexa-substituted imidazole[1,2-a] pyridine fluorescent material and a synthesis method thereof. The molecular structural formula is shown in the specification. The method comprises the following specific steps: dissolving a cyclopentadienone derivative, a 1,2-dicarbonyl compound and an ammonium salt in an anhydrous solvent, wherein the molar ratio of the cyclopentadienone derivative to the 1,2-dicarbonyl compound to ammonium salt is 1:(0.9-1.1):(4-10); heating to 100-160 DEG C to react for 2-6 hours, performing reduced pressure distillation after the reaction to remove the solvent, and purifying to obtain the solid, namely the fluorescent material 2,3,5,6,7,8-hexa-substituted imidazole[1,2-a] pyridine compound. In the method, the cyclopentadienone derivative and 1,2-dicarbonyl compound are utilized as raw materials and the inorganic ammonium salt is utilized as an ammonia source, thus the source range of the raw materials is wide, the raw materials are easily available, the operations are simple, a one-pot method is adopted for synthesis, the steps are short, the yield is high and the method can be used in mass production easily.
Owner:ZHEJIANG UNIV
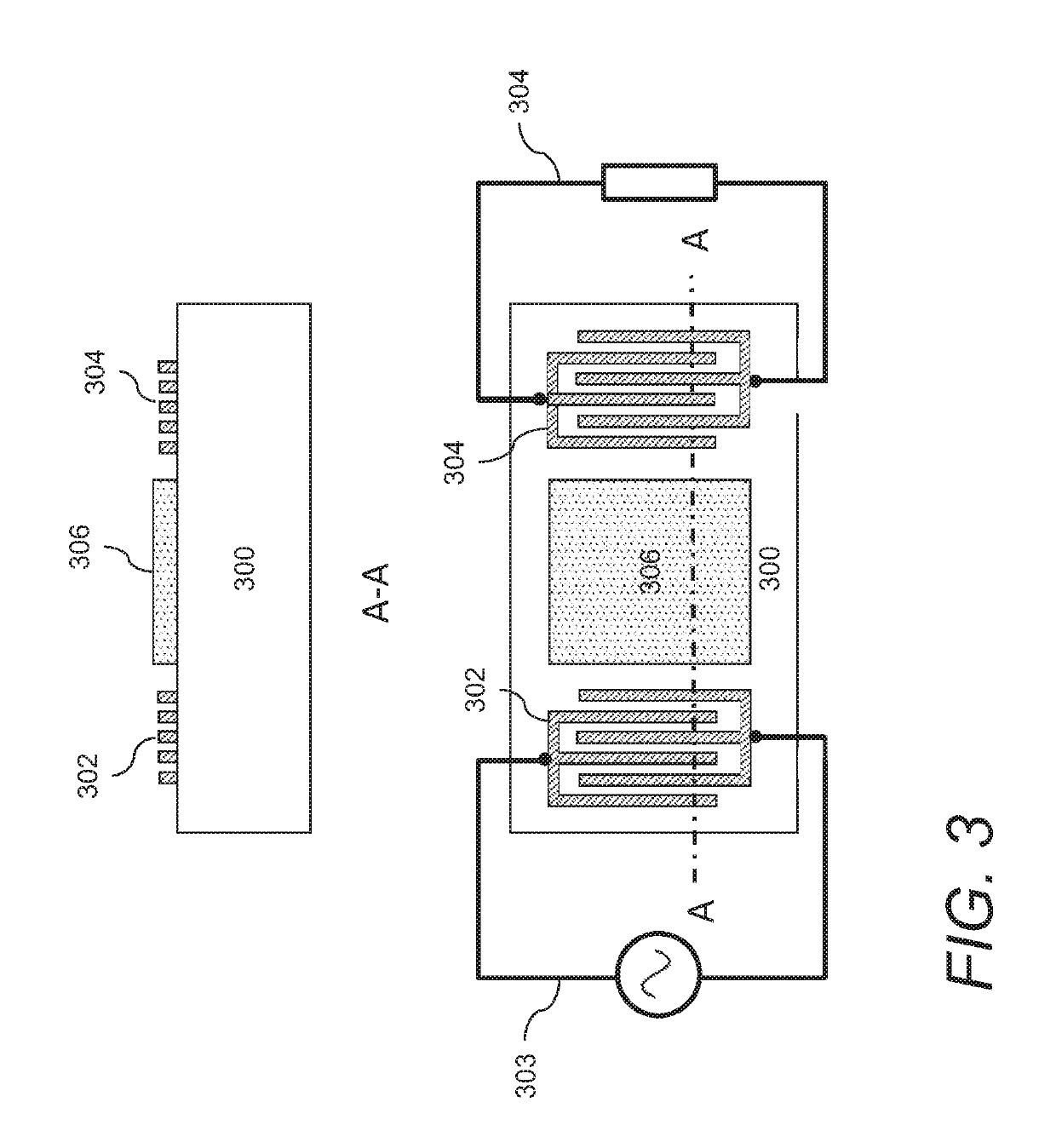
Gas sensors and methods of sensing a gas-phase analyte
PendingUS20200400629A1Change propertiesAnalysing fluids using sonic/ultrasonic/infrasonic wavesMaterial analysis using wave/particle radiationAnalyteCyclopentadienone
Gas sensors are provided. The gas sensors comprise: a substrate; a plurality of electrodes on the substrate; and a polymeric sensing layer on the substrate for adsorbing a gas-phase analyte. The adsorption of the analyte is effective to change a property of the gas sensor that results in a change in an output signal from the gas sensor. The polymeric sensing layer comprises a polymer chosen from substituted or unsubstituted polyarylenes comprising the reaction product of monomers comprising a first monomer comprising an aromatic acetylene group and a second monomer comprising two or more cyclopentadienone groups, or a cured product of the reaction product. The gas sensors and methods of using such sensors find particular applicability in the sensing of gas-phase organic analytes.
Owner:ROHM & HAAS ELECTRONICS MATERIALS LLC
Silylated polyarylenes
InactiveCN105694005AImprove compatibilityGood flexibilitySynthetic resin layered productsElectrical equipmentCyclopentadienoneAlkyne
Polyarylenes comprising as polymerized units a first monomer having two cyclopentadienone moieties and a second monomer having two or more alkyne moieties, wherein at least one alkyne moiety is directly bonded to a silicon atom are provided. Such polyarylenes are useful as dielectric materials in the manufacture of electronic devices.
Owner:ROHM & HAAS ELECTRONICS MATERIALS LLC
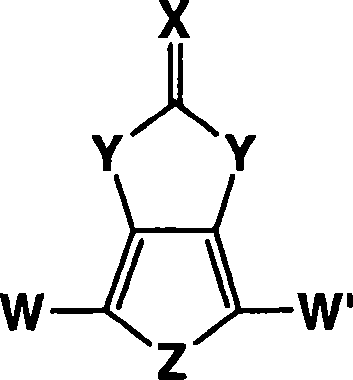
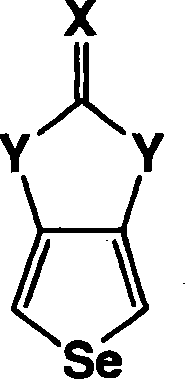
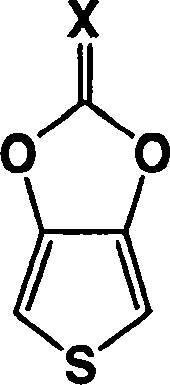
Heterocyclic fused imidazolone, dioxolone, imidazolethione and dioxolethione monomers
A method of making a compound of the formula shown below is disclosed: wherein Z is Se or S; Y is NH or O; X is O or S and W and W' are independently selected from the group consisting of hydrogen, -C=ONH 2 , -C=ONHR', -C=ONR'R', -C N, and R' is C 1-6 alkyl and the resultant monomer compounds. The method includes contacting a 3,4-disubstituted thiophene or selenophene or disubstituted thiophene or selenophene derivatives with a carbonyl or thiocarbonyl containing compound selected from the group consisting of ureas, thioureas, carbonate esters, thionocarbonates, thiocarbonate esters, or orthocarbonates. The monomer compounds are suitable for forming polymers for use in a wide range of electronic applications.
Owner:AIR PROD & CHEM INC
Acoustic wave sensors and methods of sensing a gas-phase analyte
ActiveUS20190219543A1Analysing fluids using sonic/ultrasonic/infrasonic wavesResponse signal detectionGas phasePolyamide
Acoustic wave sensors comprise: a piezoelectric layer, first and second electrodes arranged with the piezoelectric layer in a piezoelectric transducer circuit; and a polymeric sensing layer for adsorbing a gas-phase analyte, the adsorption of which analyte causes a change in resonant frequency of the piezoelectric transducer circuit, wherein the polymeric sensing layer comprises: (a) a polymer chosen from substituted or unsubstituted: polyarylenes comprising the reaction product of monomers comprising a first monomer comprising an aromatic acetylene group and a second monomer comprising a cyclopentadienone group; polyamides; polypyrazoles; or novolacs; or a cured product thereof; (b) a polymer chosen from substituted or unsubstituted: polyamic acids; or polyamic acid-polyimide copolymers; (c) a polymer formed from one or more monomers comprising a monomer comprising a polar group-substituted arylcyclobutene group, or a cured product thereof; or (d) a polymer comprising polymerized units of a monomer chosen from substituted or unsubstituted: maleimides; or norbomenes; or a cured product thereof. The acoustic wave sensors and methods of using such sensors find particular applicability in the sensing of gas-phase analytes.
Owner:ROHM & HAAS ELECTRONICS MATERIALS LLC
Building insulating material for inner wall
ActiveCN105255237AImprove insulation effectFlame resistantFireproof paintsReflecting/signal paintsEpoxyWeather resistance
The invention discloses a building insulating material for an inner wall. The building insulating material is prepared from, by weight, fluorine-containing epoxy resin, 3-diethyl amino propyl amine, bismaleimide, allylboronic acid pinacol ester, foam glass, ethylene-vinyl acetate copolymers, 3,3'-(phenylene oxide) bi(2,4,5-triphenyl) cyclopentadienone, polyazelaic polyanhydride, water, dispersing agents, thickening agents, antifoaming agents, wetting agents and mildewproof agents. When the building insulating material is used for the inner wall of a building, the insulating effect is obvious, non-flame performance and weather resistance are achieved, and the building insulating material is small in specific gravity and heat conductivity coefficient, good in adhesive performance, not prone to falling and good in tenacity; meanwhile, the density of a coating is reduced, the heat conduction coefficient of the coating is reduced, the luminous reflectance of the coating is improved, and the insulation effect is improved.
Owner:湖南中科鑫云涂料科技有限公司
Thermal-insulation paint for building external walls
InactiveCN105176316AStrong impact resistanceImprove the decorative effectEpoxy resin coatingsHigh resistanceEpoxy
The invention discloses a thermal-insulation paint for building external walls, which is prepared from the following raw materials in parts by weight: an epoxy resin, bismaleimide, di-tert alcohol propenyl borate, foam glass, an ethylene-vinyl acetate copolymer, 3,3'-(p-phenyleneoxy)bis(2,4,5-triphenyl)cyclopentadiene ketone, nano alumina-silica fiber, a dispersing agent, a thickener, a defoaming agent and a wetting agent. The paint has the advantages of low raw material loss, simple technique, hard paint film, favorable glossiness, high impact resistance, low cracking tendency, soft tactile sensation, high decorating property, high weather resistance, high scrub resistance, high adhesive force, long service life, no special smell, no heavy metals (such as mercury, lead and the like), no volatile formaldehyde, synergistic actions among substance molecules, very high stability, high adhesiveness and high film formation efficiency, enhances the density of the coating film, and improves the film formation quality. Thus, the film has the advantages of high adhesive force, no bubble or crack, high compactness, high uniformity and the like.
Owner:CHONGQING RADIO & TV UNIV +1
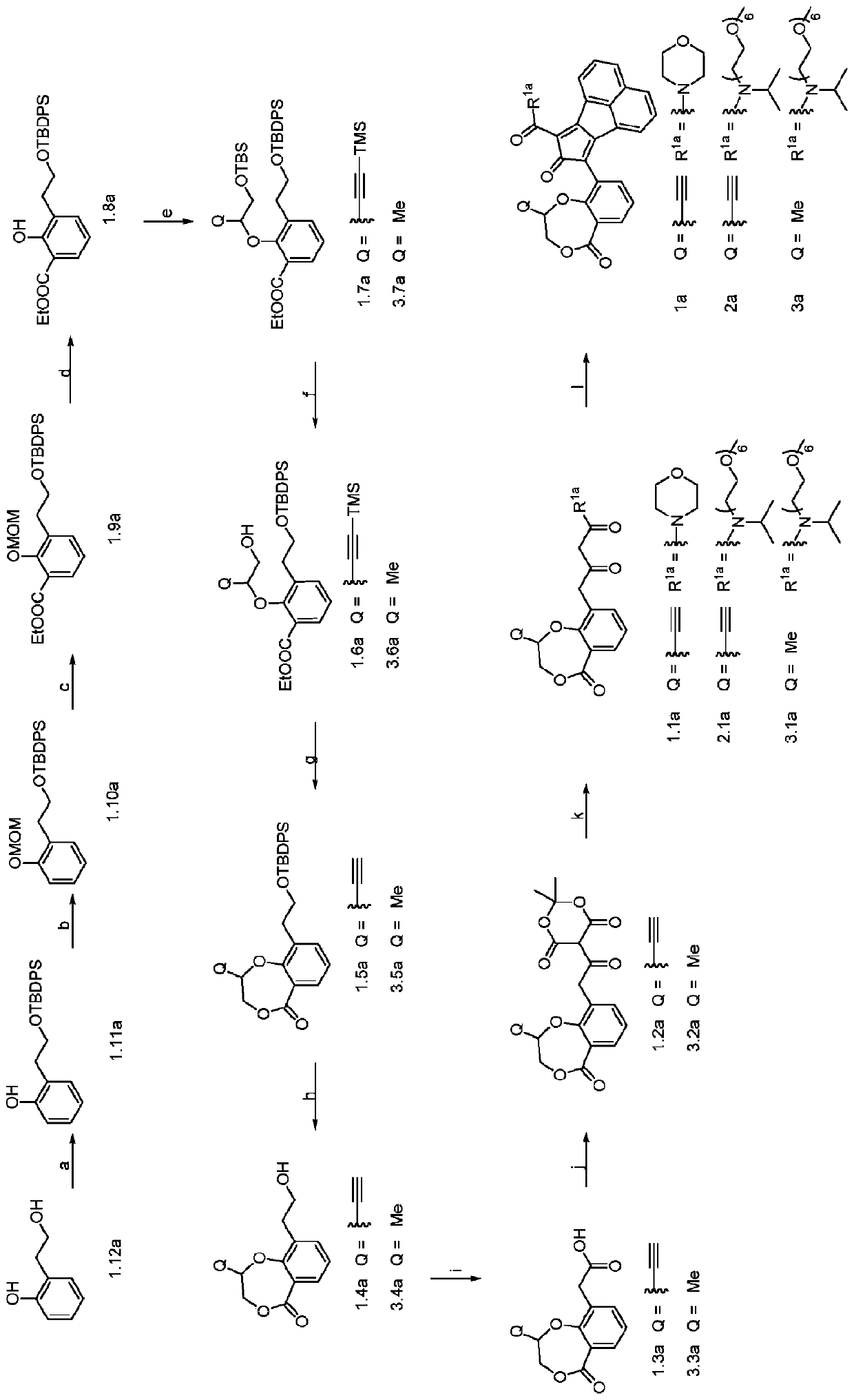
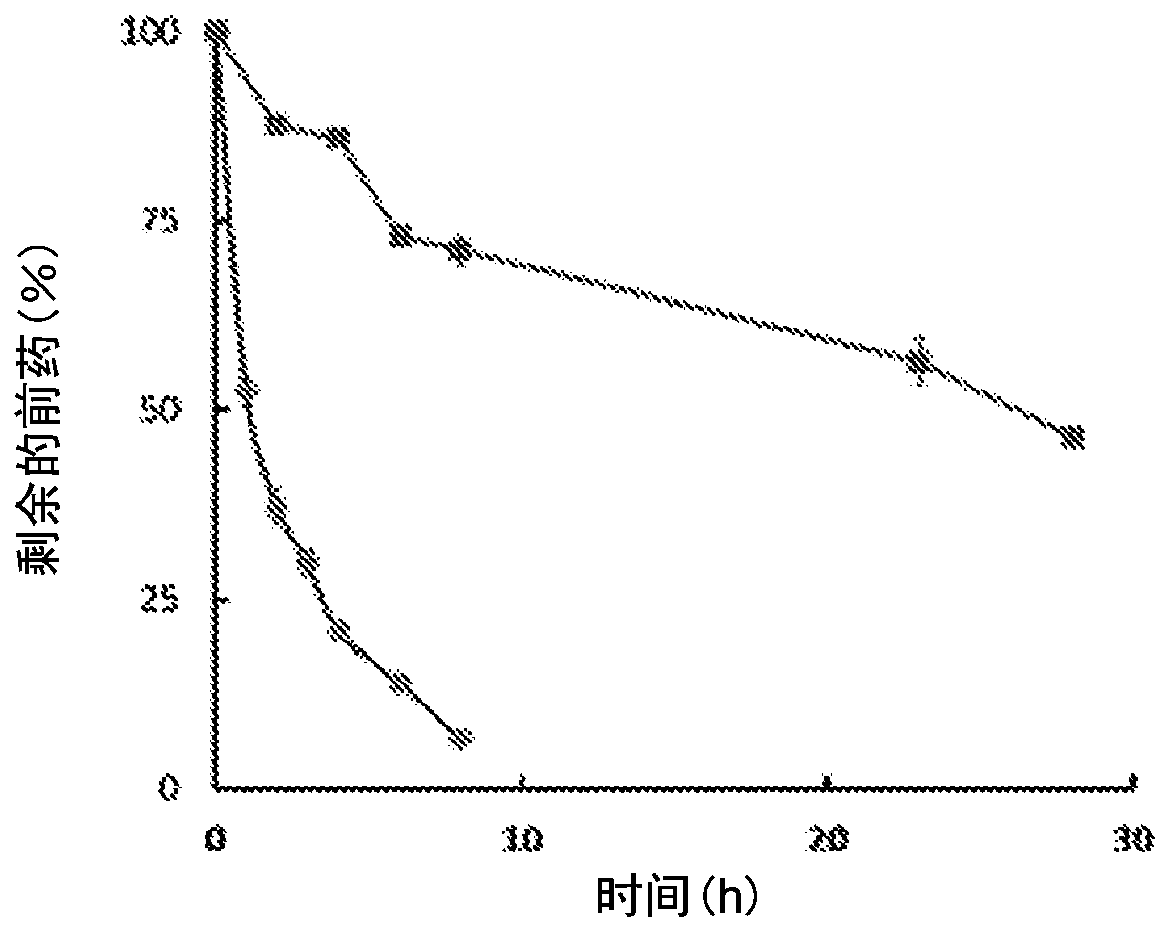
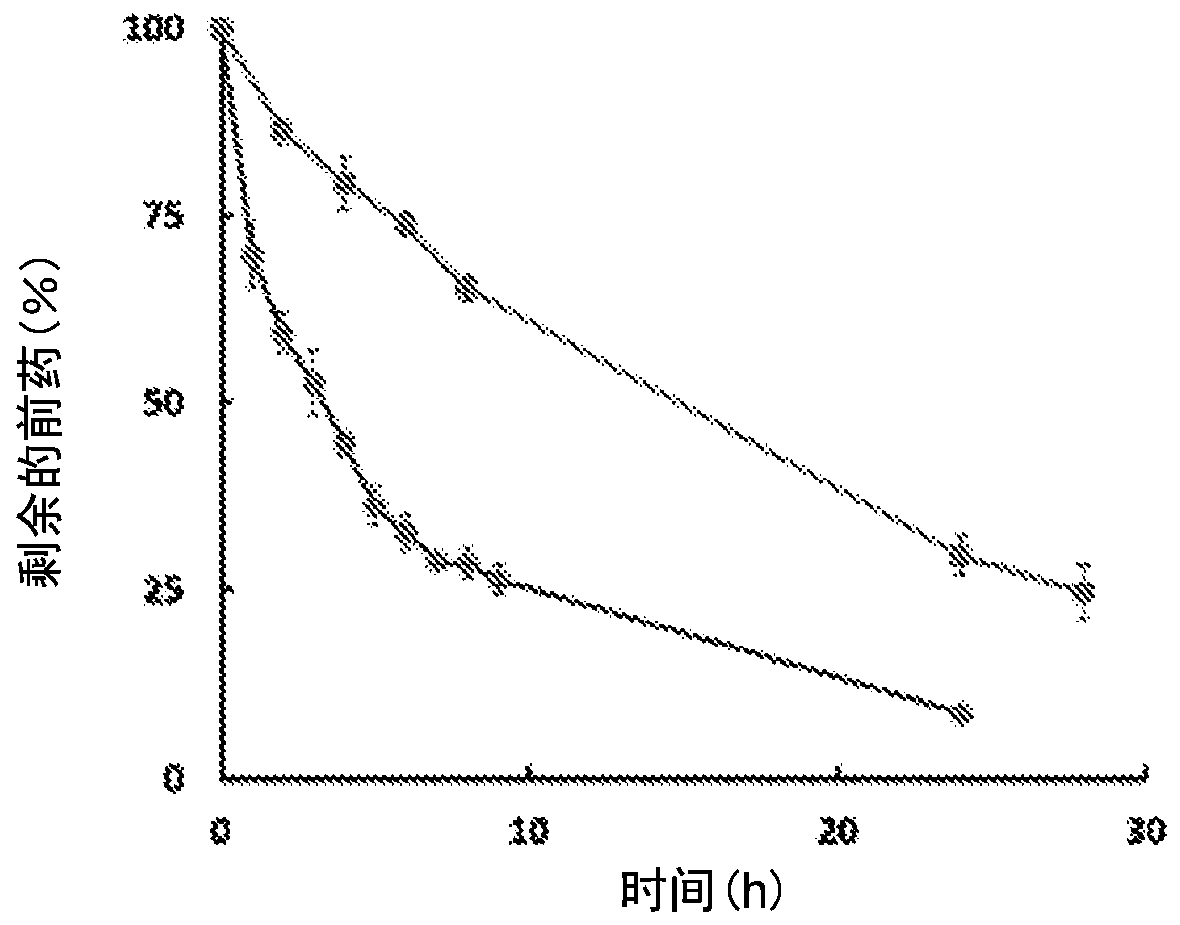
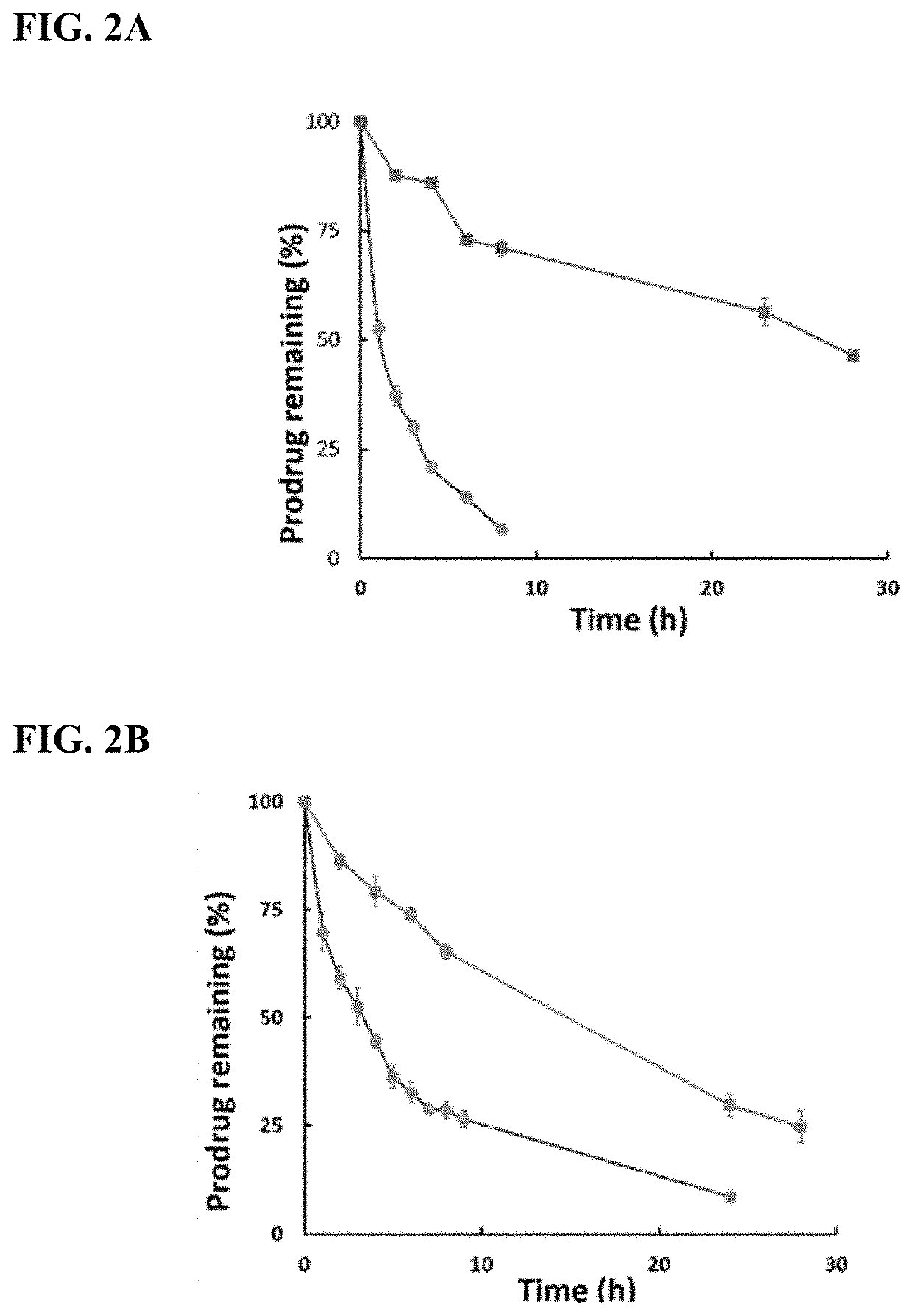
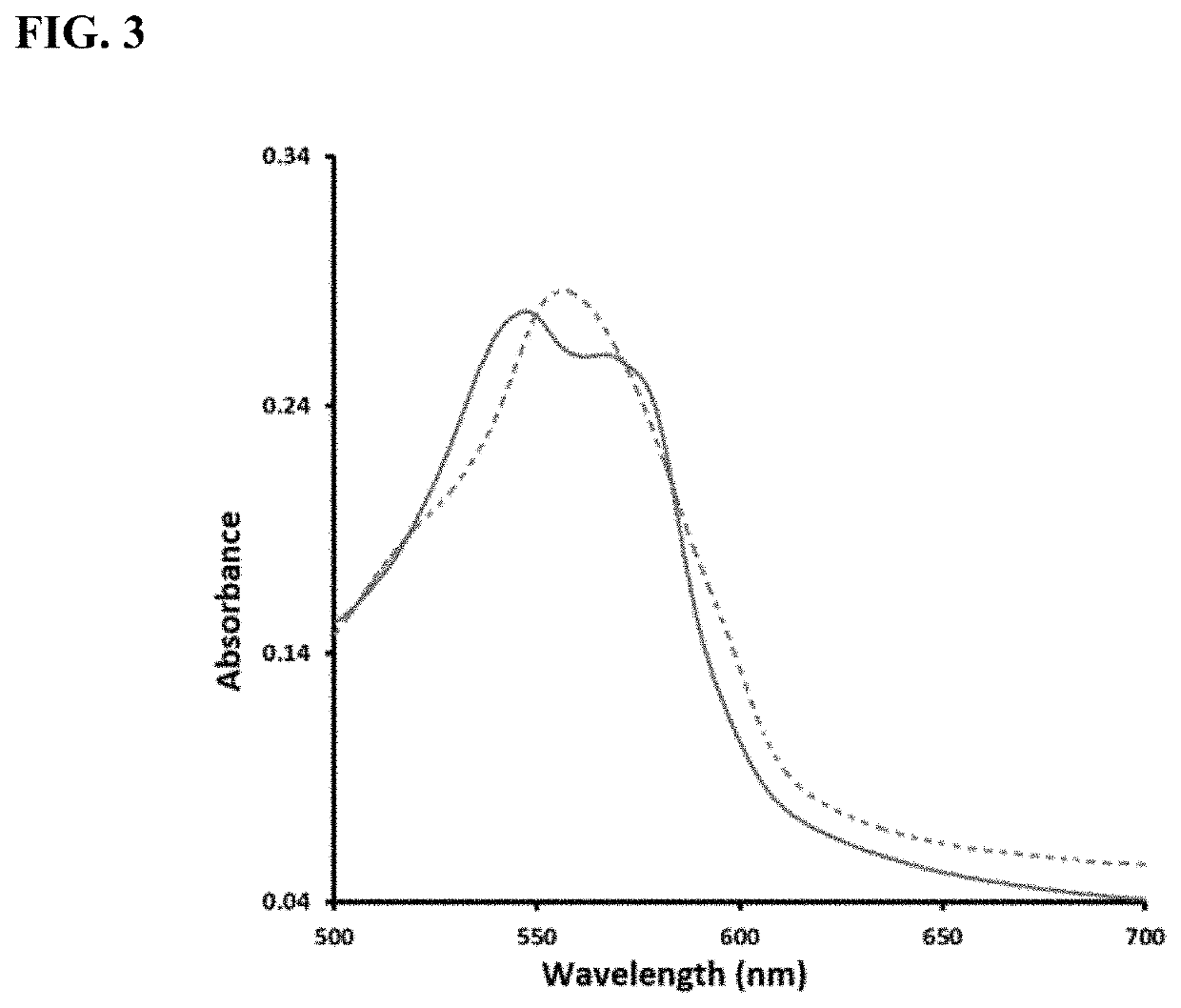
Enzyme-triggered carbon monoxide releasing molecules
The present invention generally relates to carbon monoxide releasing compounds and compositions, and their use as carbon monoxide prodrugs. The compounds disclosed herein contain a cyclopentadienone moiety, a non-reactive dienophile, and an enzyme-cleavable tethering moiety connecting the cyclopentadienone moiety to the non-reactive dienophile. Cleavage of the enzyme-cleavable tethering moiety results in conversion of the non-reactive dienophile to a reactive dienophile
Owner:GEORGIA STATE UNIV RES FOUND INC
Method for synthesizing 3,3'-(p-phenyleneoxy)di(2,4,5-triphenyl)cyclopentadiene
InactiveCN101100420BAvoid toxicityEasy to processCarbonyl compound preparation by condensationPtru catalystPhenyl Ethers
A method for synthesizing 3,3-(phenylene oxide)di(2, 4, 5-triphenyl)cyclopentadienyl ketone is carried out by Foury reacting phenyl ether with phenacerine with anhydrous aluminum muriate as catalyst and methylene dichloride as solvent to prepare 4-phenacelate withIstructure, oxidizing 4-phenacelate into 4-phenyl-oxalate withIIstructure with potassium permanganate as oxidant and under existence ofphase-transferring catalyst, and condensing 4-phenyl-oxalate withIIstructure with 1,3-diphenyl acetone under alkali catalyzing. It's cheap, efficient and non-toxic.
Owner:INST OF CHEM CHINESE ACAD OF SCI
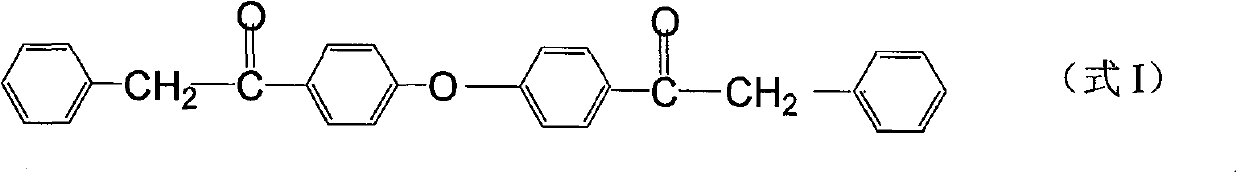
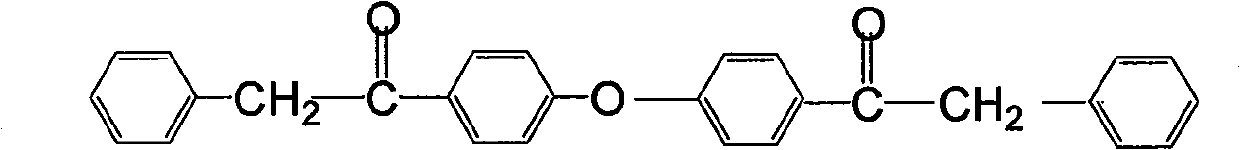
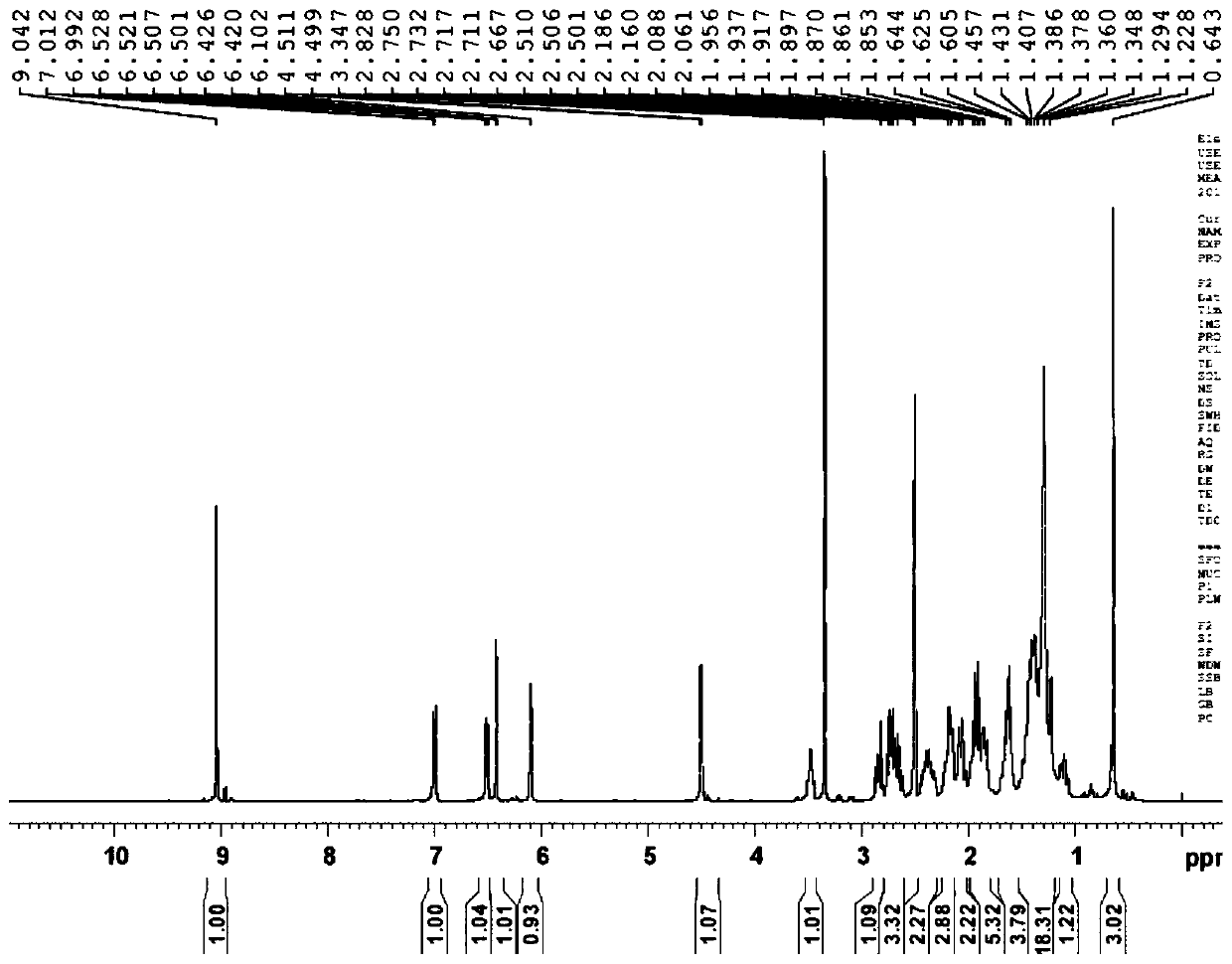
Preparation method of fulvestrant related substance E
The invention relates to a preparation method of a fulvestrant related substance E. The invention relates to a preparation method of a fulvestrant related substance E shown as a formula I (See the specification for reference), namely, 7-[9-(4, 4, 5, 5, 5-pentafluoropentylsulfinyl) nonyl] estra-1, 3, 5(10), 6-tetraene-3, 17 beta-diol. The fulvestrant related substance E is obtained by taking (7S, 8R, 9S, 13S, 14S, 17S)-3, 17-dihydroxy-13-methyl-7-(9-((4, 4, 5, 5, 5-pentafluoropentyl) sulfo) nonyl)-7, 8, 9, 11, 12, 13, 14, 15, 16, 17-decahydro-6H-cyclopentadiene-6-ketone as a starting material through the reaction steps of protection, reduction, oxidation, dehydration and deprotection. The method has the advantages of few side reactions, high product yield, good purity, reasonable process design, simple operation and low cost.
Owner:JIANGSU HANSOH PHARMA CO LTD
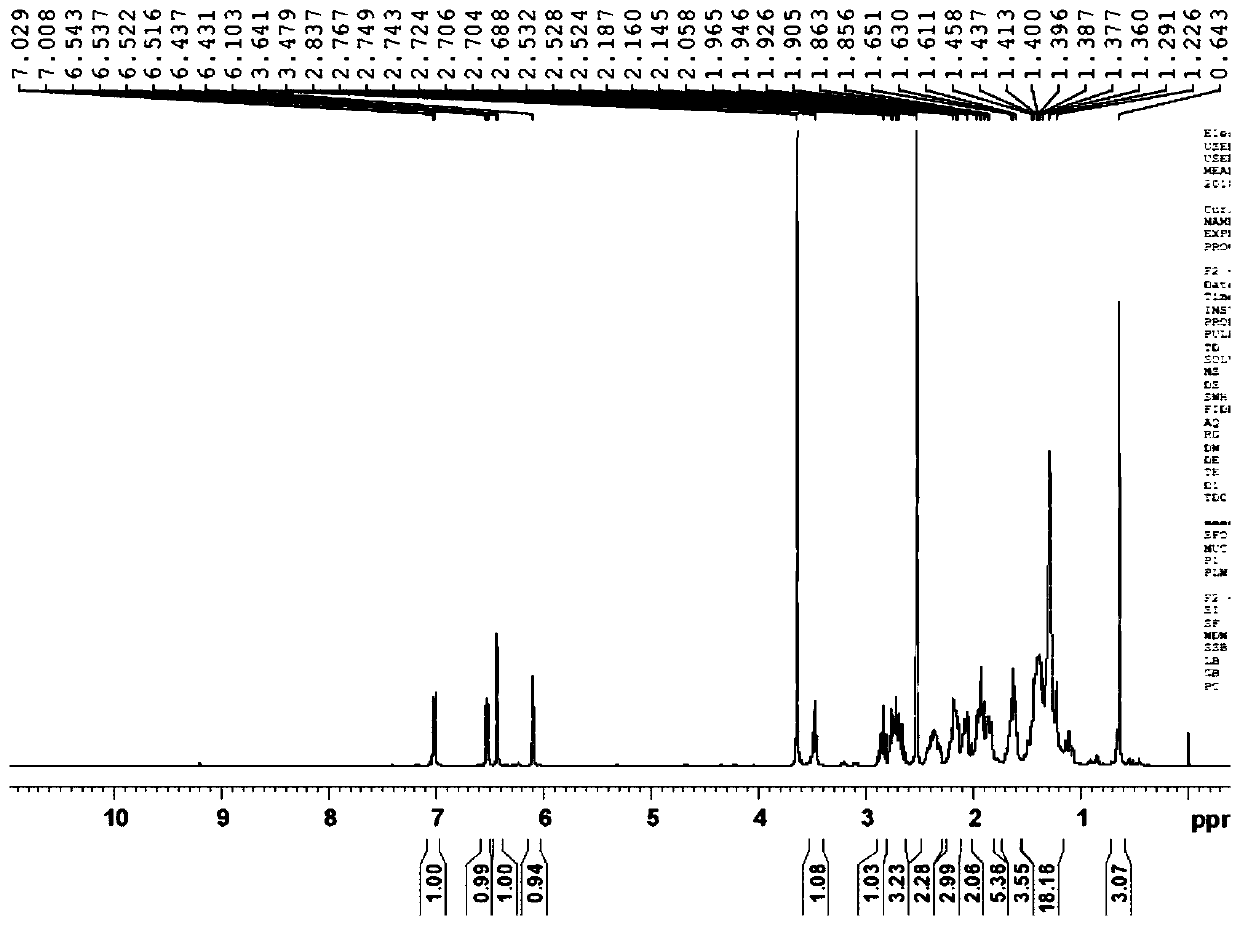
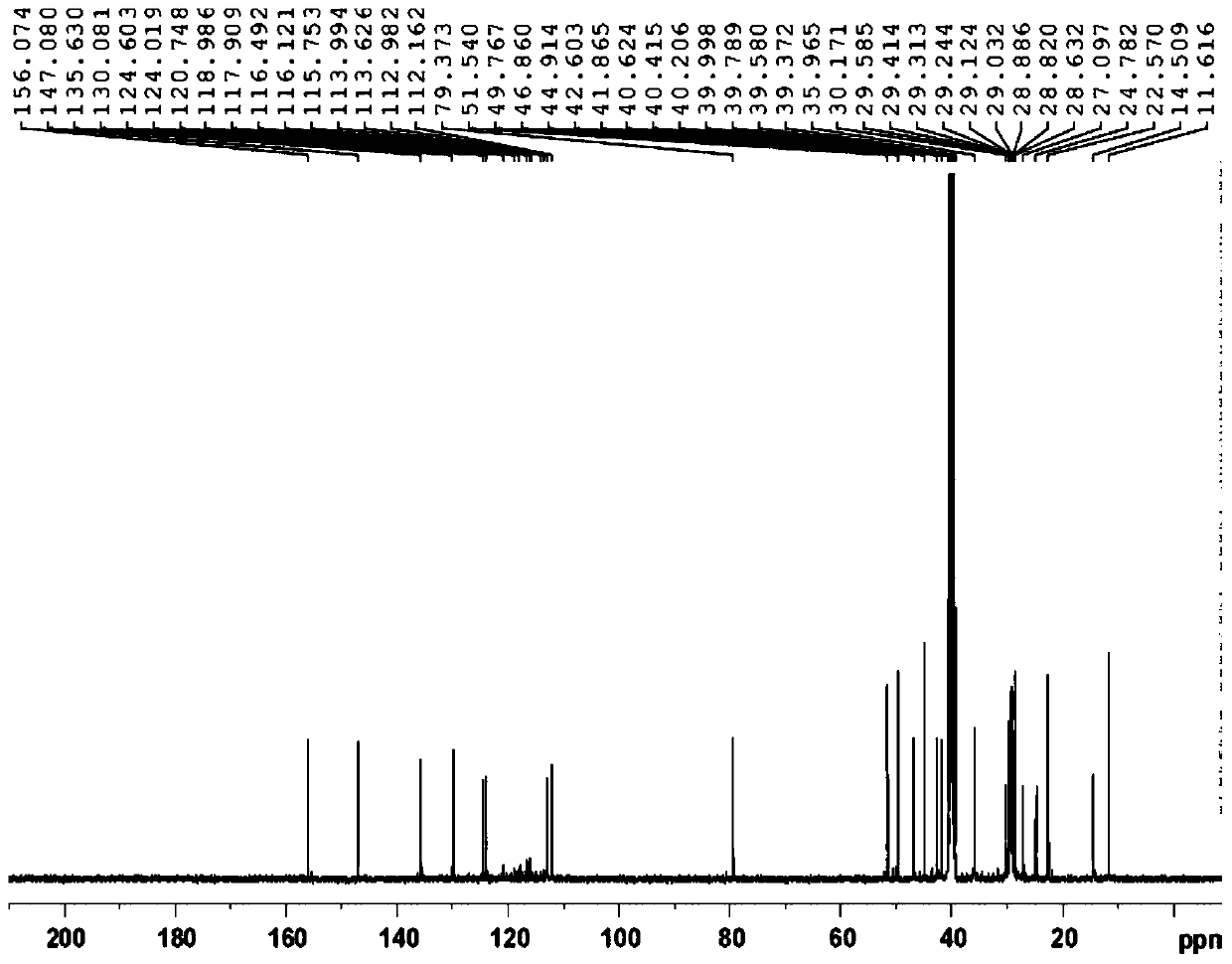
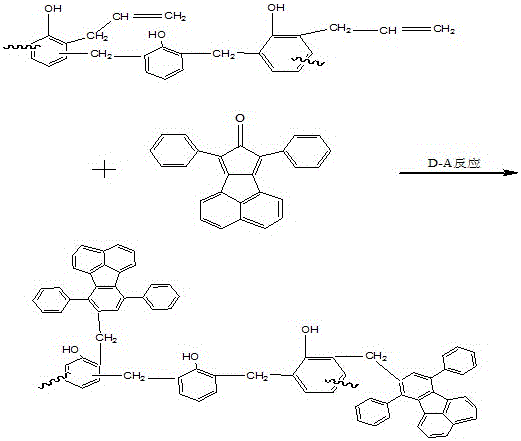
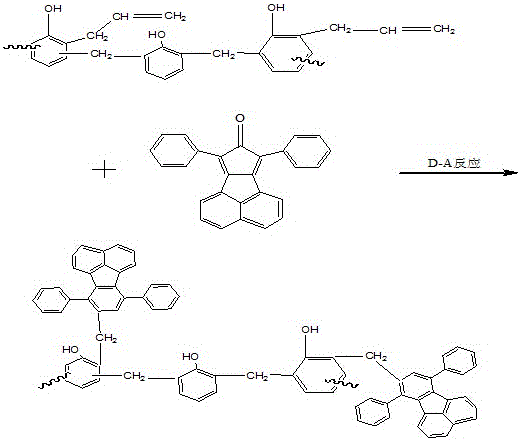
Acenaphthene type polyphenyl copolymerized allyl phenolic aldehyde active dilute resin and preparation method thereof
The invention relates to an acenaphthene type polyphenyl copolymerized allyl phenolic aldehyde active dilute resin and a preparation method of the acenaphthene type polyphenyl copolymerized allyl phenolic aldehyde active dilute resin, and relates to a resin and the preparation method of the resin. The allyl phenolic aldehyde resin is generated by the reaction of compounded phenolic resin and chloropropene; the resin generates Claisen rearrangement reaction under high temperature, and further generates allyl phenolic aldehyde resin; under the same temperature, acenaphthene type cyclopentadiene ketone and allyl resin are added to generate Diels-Alder reaction; finally, phenolic hydroxyl hydrogen in phenolic resin replaced by allyl of the acenaphthene type polyphenyl copolymerized allyl phenolic aldehyde active dilute resin is obtained; the prepared resin is relatively low in viscosity, and the resin can be used as the active dilute resin for reducing resin viscosity. Besides, an acenaphthene type polyphenyl condensed ring structure with big molecular radical group is introduced, and has Diel-Alder reaction with double keys in the allyl phenolic resin under a specific condition; multiple benzene ring groups are introduced, so that the compounded resin is possessed of high temperature resistance and thermal oxidization stability.
Owner:SHENYANG INSTITUTE OF CHEMICAL TECHNOLOGY
Method for synthesizing 3,3'-(p-phenyleneoxy)di(2,4,5-triphenyl)cyclopentadiene
InactiveCN101100420AAvoid toxicityToxicCarbonyl compound preparation by condensationPhenyl EthersCyclopentadienone
A method for synthesizing 3,3-(phenylene oxide)di(2, 4, 5-triphenyl)cyclopentadienyl ketone is carried out by Foury reacting phenyl ether with phenacerine with anhydrous aluminum muriate as catalyst and methylene dichloride as solvent to prepare 4-phenacelate withIstructure, oxidizing 4-phenacelate into 4-phenyl-oxalate withIIstructure with potassium permanganate as oxidant and under existence of phase-transferring catalyst, and condensing 4-phenyl-oxalate withIIstructure with 1,3-diphenyl acetone under alkali catalyzing. It's cheap, efficient and non-toxic.
Owner:INST OF CHEM CHINESE ACAD OF SCI
Polyarylene material
ActiveCN105384915BSemiconductor/solid-state device detailsSolid-state devicesPolymer scienceOligomer
Owner:ROHM & HAAS ELECTRONICS MATERIALS LLC
Building thermal insulation materials for interior walls
ActiveCN105255237BImprove insulation effectFlame resistantFireproof paintsReflecting/signal paintsEpoxyBuilding insulation materials
The invention discloses a building thermal insulation material for interior walls. The raw materials of the thermal insulation coating include fluorinated epoxy resin, 3-diethylaminopropylamine, bismaleimide, acryl boric acid ortho Di-tertiary alcohol ester, foam glass, ethylene-vinyl acetate copolymer, 3,3'-(p-phenylene oxide)bis(2,4,5-triphenyl)cyclopentadienone, polyazelaic anhydride, Water, dispersant, thickener, defoamer, wetting agent, antifungal agent; used in building interior wall insulation effect is obvious, with flame resistance, weather resistance, small specific gravity, small thermal conductivity, good bonding performance , not easy to fall off, good toughness, while reducing the density of the coating, reducing the thermal conductivity of the coating, increasing the light reflectivity of the coating, and improving the heat insulation effect.
Owner:湖南中科鑫云涂料科技有限公司
A kind of acenaphthyl polyphenyl copolymerized allyl phenolic reactive diluent resin and preparation method thereof
An acenaphthene-type polyphenyl copolymerized allyl phenolic active dilution resin and a preparation method thereof, relating to a resin and a preparation method thereof, the phenolic resin synthesized by the present invention reacts with allyl chloride to generate an allyl ether phenolic resin, and the resin is used in Claisen rearrangement reaction occurs at high temperature, and then allyl phenolic resin is generated; at the same temperature, acenaphthyl cyclopentadienone is added to undergo Diels-Alder reaction with allyl resin, and finally acenaphthyl polyphenyl copolymerized allyl The phenolic reactive diluting resin replaces the phenolic hydroxyl hydrogen in the phenolic resin with an allyl group, and the resulting resin has a relatively low viscosity, which can be used as a reactive diluting resin to reduce the resin viscosity. The acenaphthene-type polyphenyl condensed ring structure with larger molecular groups is introduced, and under specific conditions, Diels-Alder reaction occurs with the double bond in the allyl phenolic resin, and multiple benzene ring groups are introduced to make the synthesized resin It has high temperature resistance and thermo-oxidative stability.
Owner:SHENYANG INSTITUTE OF CHEMICAL TECHNOLOGY
2,3,5,6,7,8-hexa-substituted imidazole[1,2-a] pyridine fluorescent material and synthesis method thereof
InactiveCN102286278BRaw materials are easy to getEasy to operateOrganic chemistryLuminescent compositionsFluorescenceSynthesis methods
The invention discloses a 2,3,5,6,7,8-hexa-substituted imidazole[1,2-a] pyridine fluorescent material and a synthesis method thereof. The molecular structural formula is shown in the specification. The method comprises the following specific steps: dissolving a cyclopentadienone derivative, a 1,2-dicarbonyl compound and an ammonium salt in an anhydrous solvent, wherein the molar ratio of the cyclopentadienone derivative to the 1,2-dicarbonyl compound to ammonium salt is 1:(0.9-1.1):(4-10); heating to 100-160 DEG C to react for 2-6 hours, performing reduced pressure distillation after the reaction to remove the solvent, and purifying to obtain the solid, namely the fluorescent material 2,3,5,6,7,8-hexa-substituted imidazole[1,2-a] pyridine compound. In the method, the cyclopentadienone derivative and 1,2-dicarbonyl compound are utilized as raw materials and the inorganic ammonium salt is utilized as an ammonia source, thus the source range of the raw materials is wide, the raw materials are easily available, the operations are simple, a one-pot method is adopted for synthesis, the steps are short, the yield is high and the method can be used in mass production easily.
Owner:ZHEJIANG UNIV
Enzyme-triggered carbon monoxide releasing molecules
The present invention generally relates to carbon monoxide releasing compounds and compositions, and their use as carbon monoxide prodrugs. The compounds disclosed herein contain a cyclopentadienone moiety, a non-reactive dienophile, and an enzyme-cleavable tethering moiety connecting the cyclopentadienone moiety to the non-reactive dienophile. Cleavage of the enzyme-cleavable tethering moiety results in conversion of the non-reactive dienophile to a reactive dienophile.
Owner:GEORGIA STATE UNIV RES FOUND INC
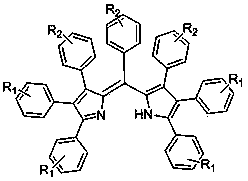
Pyrrole methenyl fluorescent dye and preparation method thereof
InactiveCN102558898BEasy to separate and purifyWide range of sourcesMethine/polymethine dyesLuminescent compositionsPyrroleCyclopentenone
The invention relates to a preparation method of a pyrrole methenyl fluorescent dye. Cyclopentenone derivant and ammonium salt with the mole ratio of 1:4-200 are put in a seal tube reactor or an autoclave, the seal tube reactor or the autoclave is sealed and heated after an organic solvent is added, and reaction is carried out for 2-24 hours at the temperature of 100-140 DEG C and 1-10 atmospheres. Compared with the existing synthesis method of deriving from aromatic aldehyde and pyrrole, easy-to-make and easy-to-obtain carbonyl compound-cyclopentenone derivant and inorganic ammonium salt are adopted as raw materials, and compared with the conventional pyrrole preparation way, the preparation method disclosed by the invention has the advantages that the raw material source scope is wide, the whole preparation step is short, the operation is simple, the production rate is high, synthesis steps of protection and deprotection are avoided, multiple substituents can be obtained in one step, and products are easier to separate and purify.
Owner:ZHEJIANG UNIV
A kind of preparation method of 3,4-bis(3,4,5-trifluorophenyl)-2,5-diphenylcyclopentadienone
InactiveCN104151150BEasy to separate and purifyThe synthesis process is simpleOrganic compound preparationCarbonyl compound preparation by oxidationDibenzyl ketoneSolvent
Owner:SHANDONG UNIV
Thiophene and pyrazine derivatives and preparation method thereof
InactiveCN101830910BImprove solubilityGood light and heat stabilityOrganic chemistryLuminescent compositionsSynthesis methodsFluorescence
The invention relates to thiophene and pyrazine derivatives and a preparation method thereof, belonging to the field of organic electroluminescent materials. The derivatives are prepared by forming a molecular luminous core by centrosymmetrically connecting thiophene and pyrazine with four benzene rings, connecting functional groups of different structures and different conjugated degrees with the periphery of the core so that the red fluorescent molecules have charge-transmitting capability and can be used as a main red luminous material in the field of organic electroluminescence. The synthesis method comprises the following steps of: using 2,5-dibromothiophene as a raw material; nitrifying, carrying out C-C coupling with bromobenzene boric acid; reducing nitro groups; synthesizing a thiophene and pyrazine core with four peripheral bromines periphery together with 4,4'-dibromo benzyl; and then, carrying out C-N, C-C coupling reactions with arylamine, and the like or introducing acetylene groups to the periphery of the core and carrying out Diels-Alder cycloaddition with cyclopentadiene ketene with functional substituted groups to prepare target derivatives. The derivatives have strong absorption capability in an ultraviolet-visible light region, the dilute solution thereof emits strong fluorescence, and luminous peaks are between 600nm and 650nm.
Owner:DALIAN UNIV OF TECH
Silylated polyarylene
InactiveCN105694005BImprove compatibilityGood flexibilitySynthetic resin layered productsElectrical equipmentPolymer scienceCyclopentadienone
Owner:ROHM & HAAS ELECTRONICS MATERIALS LLC
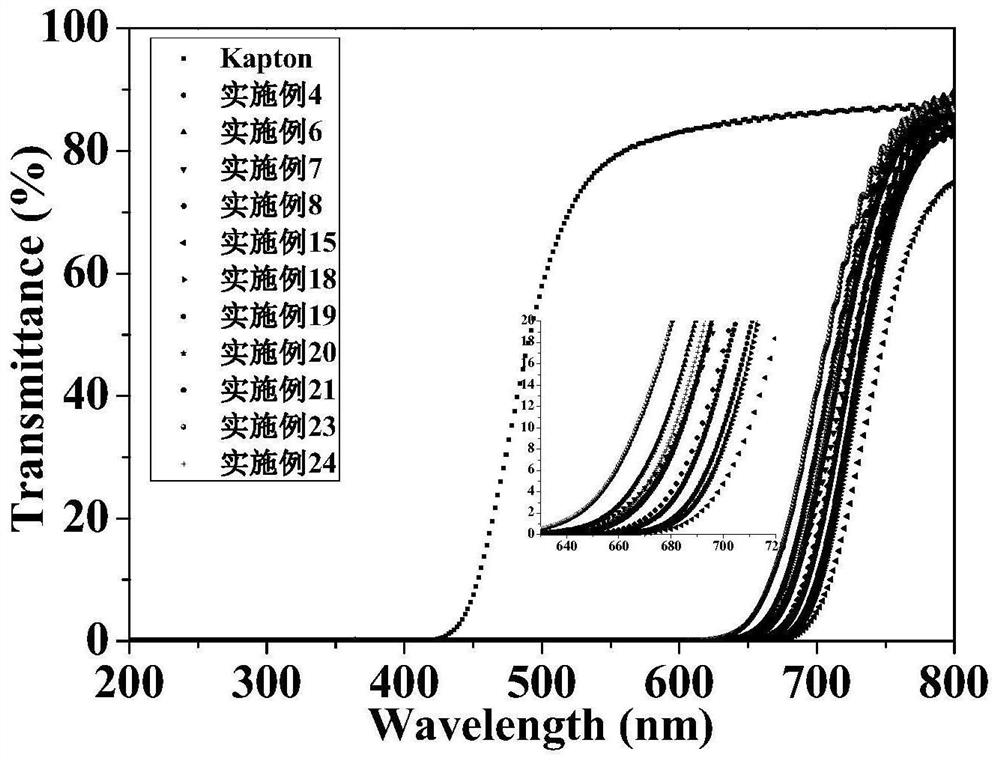
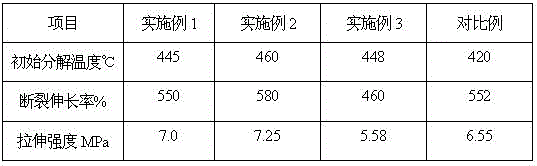
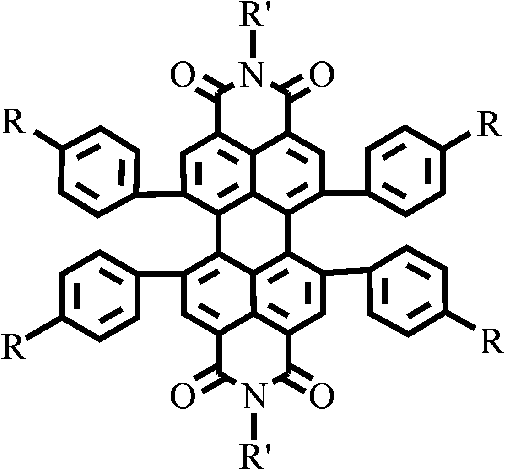
Intrinsic black polyimide as well as preparation method and application thereof
The invention discloses intrinsic black polyimide as well as a preparation method and application thereof. According to the intrinsic black polyimide, a diamine monomer and a tetracid dianhydride monomer which have large conjugation characteristics and contain a tetraphenyl cyclopentadienone structure are adopted as raw materials, polyamic acid is obtained through polymerization, and polyimide is obtained through imidization. The diamine monomer containing the tetraphenylcyclopentadienone structure and having the large conjugation characteristic has wide strong absorption in a visible light region, and when the diamine monomer is introduced into a polyimide molecular chain, polyimide can be endowed with excellent visible light absorption capacity, so that the intrinsic black characteristic is presented; such polyimides also have excellent solubility, heat resistance, electrical insulation properties and mechanical properties. The synthesis process is simple and is suitable for industrial production. The prepared intrinsic black polyimide has excellent visible light shielding performance, mechanical performance and electrical insulation performance, and can be widely applied to the fields of microelectronics, photoelectricity, military industry, aerospace and the like.
Owner:HUNAN UNIV OF TECH
Method for preparing phenanthrene-type (polyphenyl) phenyl polyhedral polyvinylsilicone rubber
InactiveCN106543730AImprove thermal stabilityImprove mechanical propertiesPolymer scienceDecomposition
The invention discloses a method for preparing phenanthrene-type (polyphenyl) phenyl polyhedral polyvinylsilicone rubber and relates to a method for preparing silicone rubber. The method comprises that vinyl polyhedral oligomeric silsesquioxane and phenanthroline-type cyclopentadienone undergo a Diels-Alder reaction to produce phenanthrene-type (polyphenyl) phenyl polyhedral polyvinylsilicone oil, and the phenanthrene-type (polyphenyl) phenyl polyhedral polyvinylsilicone oil and silicone rubber undergo a reaction to produce the phenanthrene-type (polyphenyl) phenyl polyhedral polyvinylsilicone rubber. The phenanthrene-type (polyphenyl) phenyl polyhedral polyvinylsilicone rubber has a high conjugation degree condensed ring group and an inserted polyhedral oligomeric silsesquioxane inorganic framework structure and the two structures cooperate. The heat-resistant additive has the high conjugation degree condensed ring group (containing multiple benzene ring groups) and the inserted polyhedral oligomeric silsesquioxane inorganic framework structure and the two structures cooperate so that the thermal stability and mechanical properties of the silicone rubber are greatly improved. The initial decomposition temperature is 460 DEG C, tensile strength is greater than 5Mpa and breaking elongation is 300%.
Owner:SHENYANG INSTITUTE OF CHEMICAL TECHNOLOGY
Synthesis method of indenofluorene derivatives, isotruxene and mono-substituted isotruxene derivatives
InactiveCN102627522BOrganic compound preparationHydrocarbon from oxygen organic compoundsEthyl groupKetone
The invention discloses a synthesis method of indenofluorene derivatives, isotruxene and mono-substituted isotruxene derivatives. The synthesis method of indenofluorene derivatives is characterized in that ester-group-containing compounds are formed through Diels-Alder reaction of methyl propiolate and indenocyclopentadienone, ethyl substituted indenofluorene derivatives are formed through hydrolysis, acid catalytic ring closing, carbonyl reduction and introduction of ethyl onto methylene, diketone compounds produced after ring closing react with lithium salt of 4, 4'-di-tert-butyl-2-brominated biphenyl, and then acidification and ring closing are conducted to obtain indenofluorene derivatives with spirofluorene. The synthesis method of isotruxene is characterized in that 1, 4-diphenyl-2, 3-di (carbalkoxy) fluorenone products are formed through the Diels-Alder reaction of indenocyclopentadienone and dimethyl acetylenedicarboxylate, isotruxene ketone is obtained through hydrolysis and acid catalytic ring closing and finally isotruxene is obtained; dibenzyl alcohol products are formed through the Diels-Alder reaction of 1, 4-butynediol and indenocyclopentadienone, and isotruxene is obtained through acetone protection, carbonyl reduction, acetone removal and polyphosphoric acid (PPA) ring closing; and oriented oxy substituted products, i.e. isotruxene ketone which is derived from isotruxene with methylene at a No.5 position being substituted, and corresponding diethyl substituted products are additionally obtained. The indenofluorene derivatives, the isotruxene and the mono-substituted isotruxene derivatives disclosed by the invention can be used in the field of organic electroluminescence and organic micro-molecule solar cells.
Owner:EAST CHINA NORMAL UNIV
Acoustic wave sensors and methods of sensing a gas-phase analyte
ActiveUS10794866B2Analysing fluids using sonic/ultrasonic/infrasonic wavesResponse signal detectionPolyamideCopolymer
Acoustic wave sensors comprise: a piezoelectric layer, first and second electrodes arranged with the piezoelectric layer in a piezoelectric transducer circuit; and a polymeric sensing layer for adsorbing a gas-phase analyte, the adsorption of which analyte causes a change in resonant frequency of the piezoelectric transducer circuit, wherein the polymeric sensing layer comprises: (a) a polymer chosen from substituted or unsubstituted: polyarylenes comprising the reaction product of monomers comprising a first monomer comprising an aromatic acetylene group and a second monomer comprising a cyclopentadienone group; polyamides; polypyrazoles; or novolacs; or a cured product thereof; (b) a polymer chosen from substituted or unsubstituted: polyamic acids; or polyamic acid-polyimide copolymers; (c) a polymer formed from one or more monomers comprising a monomer comprising a polar group-substituted arylcyclobutene group, or a cured product thereof; or (d) a polymer comprising polymerized units of a monomer chosen from substituted or unsubstituted: maleimides; or norbomenes; or a cured product thereof. The acoustic wave sensors and methods of using such sensors find particular applicability in the sensing of gas-phase analytes.
Owner:ROHM & HAAS ELECTRONICS MATERIALS LLC
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com


![A 2,3,5,6,7,8-hexa-substituted imidazo[1,2-a]pyridine fluorescent material and its synthesis method A 2,3,5,6,7,8-hexa-substituted imidazo[1,2-a]pyridine fluorescent material and its synthesis method](https://images-eureka.patsnap.com/patent_img/0f05677d-eeee-44a4-aa21-a7971ce51982/DEST_PATH_IMAGE005.png)
![A 2,3,5,6,7,8-hexa-substituted imidazo[1,2-a]pyridine fluorescent material and its synthesis method A 2,3,5,6,7,8-hexa-substituted imidazo[1,2-a]pyridine fluorescent material and its synthesis method](https://images-eureka.patsnap.com/patent_img/0f05677d-eeee-44a4-aa21-a7971ce51982/DEST_PATH_IMAGE007.png)
![A 2,3,5,6,7,8-hexa-substituted imidazo[1,2-a]pyridine fluorescent material and its synthesis method A 2,3,5,6,7,8-hexa-substituted imidazo[1,2-a]pyridine fluorescent material and its synthesis method](https://images-eureka.patsnap.com/patent_img/0f05677d-eeee-44a4-aa21-a7971ce51982/22595DEST_PATH_IMAGE006.png)
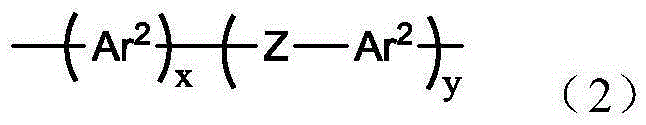
![2,3,5,6,7,8-hexa-substituted imidazole[1,2-a] pyridine fluorescent material and synthesis method thereof 2,3,5,6,7,8-hexa-substituted imidazole[1,2-a] pyridine fluorescent material and synthesis method thereof](https://images-eureka.patsnap.com/patent_img/357aa3fe-349c-4b7b-a772-d1c6af47caab/2011101587029100002DEST_PATH_IMAGE007.PNG)
![2,3,5,6,7,8-hexa-substituted imidazole[1,2-a] pyridine fluorescent material and synthesis method thereof 2,3,5,6,7,8-hexa-substituted imidazole[1,2-a] pyridine fluorescent material and synthesis method thereof](https://images-eureka.patsnap.com/patent_img/357aa3fe-349c-4b7b-a772-d1c6af47caab/22595DEST_PATH_IMAGE006.PNG)
![2,3,5,6,7,8-hexa-substituted imidazole[1,2-a] pyridine fluorescent material and synthesis method thereof 2,3,5,6,7,8-hexa-substituted imidazole[1,2-a] pyridine fluorescent material and synthesis method thereof](https://images-eureka.patsnap.com/patent_img/357aa3fe-349c-4b7b-a772-d1c6af47caab/23918DEST_PATH_IMAGE006.PNG)