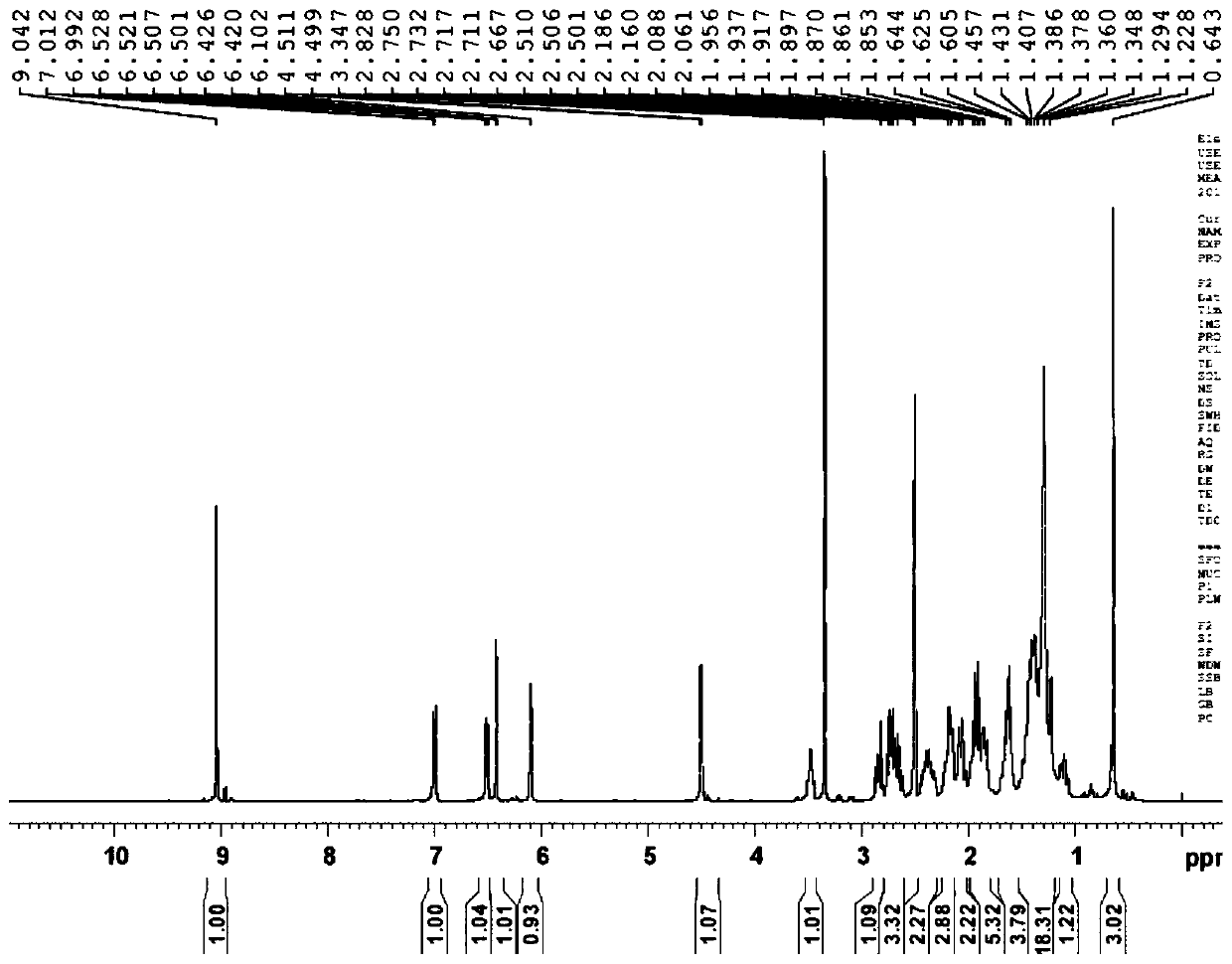
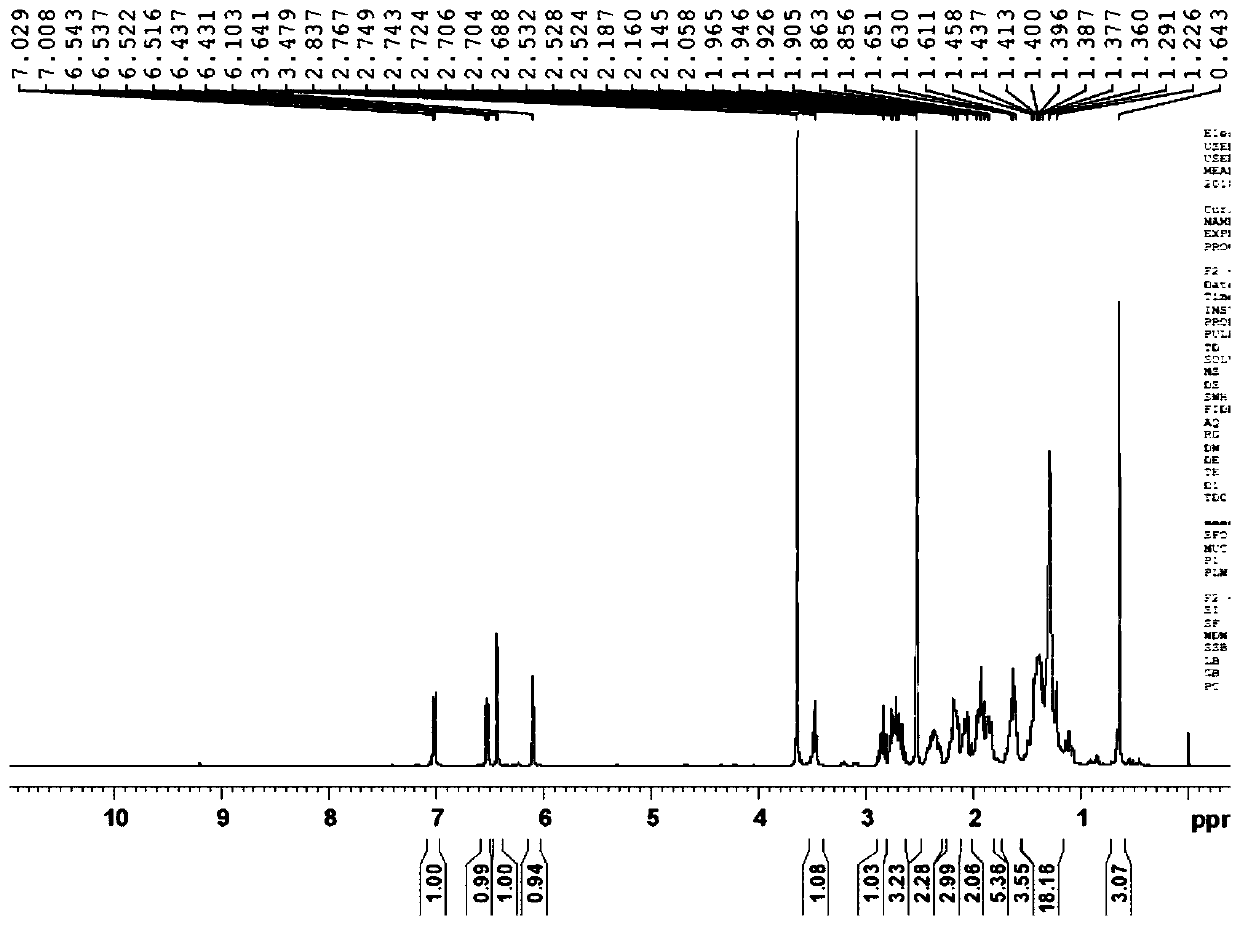
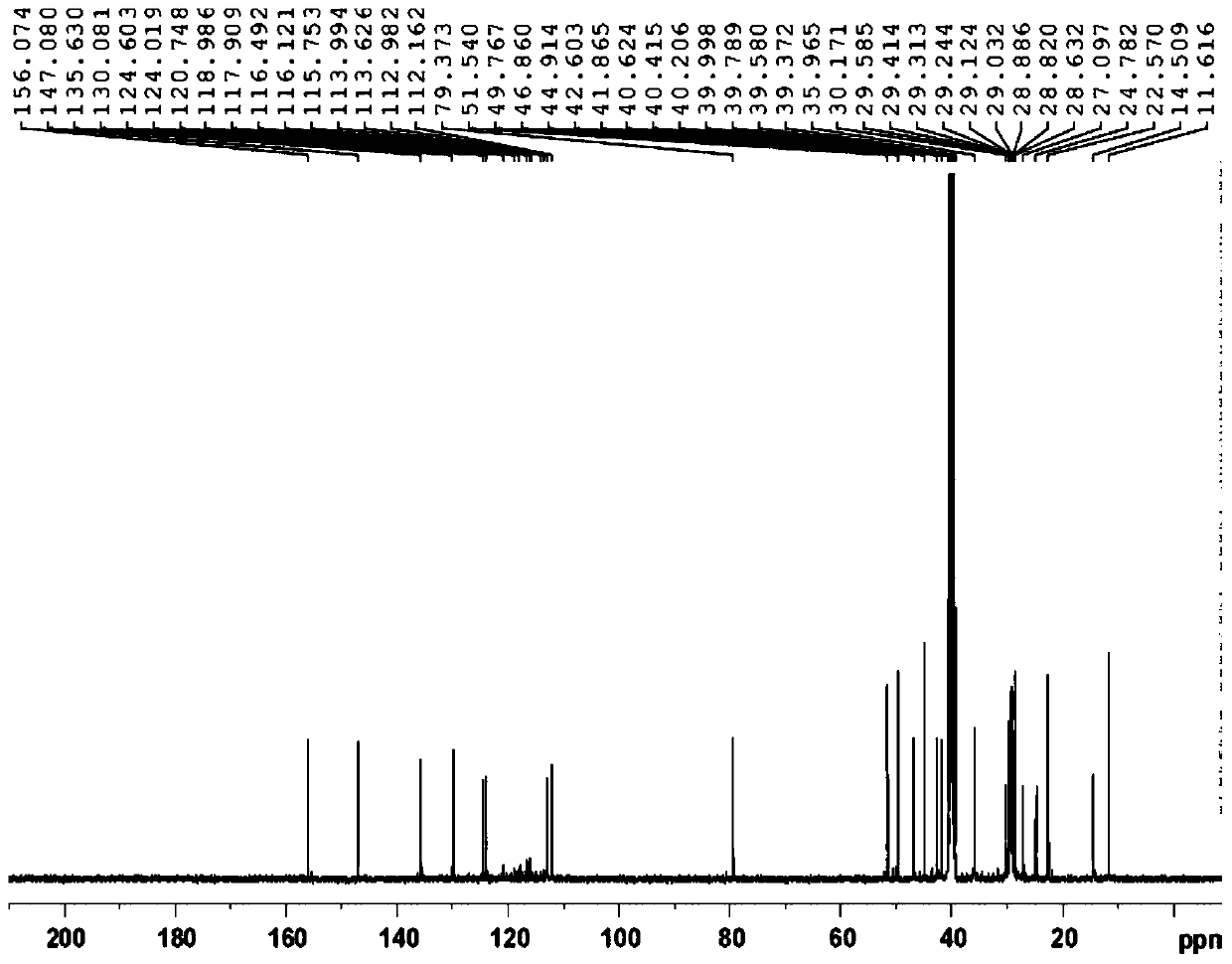
Preparation method of fulvestrant related substance E
A compound and reaction technology, applied in the field of preparation of fulvestrant related substance E, can solve the problems of easy generation of epoxy related substances, difficult reuse of resin, unstable double bond, etc., and achieves reasonable process route design and low cost. , the effect of easy operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] Preparation of compound of formula V
[0033]
[0034] The compound of formula VI (10 g, 16.5 mmol), pyridine (40 mL), and acetic anhydride (16.9 g, 165 mmol) were added into the reaction flask, and refluxed for 1 hour. The reaction solution was lowered to room temperature, added 100ml of water, 100ml of ethyl acetate, stirred and separated, the organic layer was washed successively with 100ml of saturated sodium dihydrogen phosphate, and 100ml of saturated brine, and the organic layer was dried over anhydrous sodium sulfate for 1 hour, filtered and concentrated to dryness , to obtain 11.2 g of the compound of formula V (16.3 mmol, molar yield: 98.8%).
Embodiment 2
[0036] Preparation of Formula IV Compounds
[0037]
[0038] The compound of formula V (11.2g, 16.3mmol) was added into 60mL of methanol, the temperature was lowered to -5-5°C, sodium borohydride (1.8g, 49mmol) was added in batches, and the reaction was kept for 1 hour. Pour the reaction solution into 2M hydrochloric acid aqueous solution (60ml), add 100ml of dichloromethane, stir and separate the layers, wash the organic layer with 100ml of saturated sodium bicarbonate and 100ml of saturated brine, dry the organic layer with anhydrous sodium sulfate for 1 hour, and filter After concentrating to dryness, 11.1 g (16.1 mmol, molar yield: 98.7%) of the compound of formula IV was obtained.
Embodiment 3
[0040] Preparation of the compound of formula III
[0041]
[0042] Compound of formula IV (11g, 15.9mmol), acetic anhydride (5.7g, 95.4mmol) and ethyl acetate (60ml) were added in the reaction flask, 15% hydrogen peroxide (7ml) was added dropwise, and the reaction was stirred for 20 hours after the dripping was completed. Add 7.5% sodium sulfite solution (50ml) and saturated sodium bicarbonate solution (100ml) to the reaction solution, stir for 20 minutes, separate layers, wash the organic layer with 100ml saturated brine, dry the organic layer with anhydrous sodium sulfate for 1 hour, filter and concentrate to dryness , to obtain 10.4 g (14.7 mmol, molar yield: 92.5%) of the compound of formula III.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com