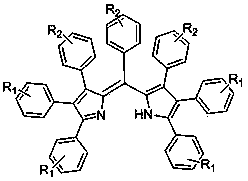
Pyrrole methenyl fluorescent dye and preparation method thereof
A fluorescent dye, pyrromethine technology, applied in the field of pyrromethine fluorescent dyes and its preparation, can solve the problems of complicated separation and purification, difficult to obtain raw materials, and low product yield, and achieve simple operation, easier separation and purification, and short preparation steps Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
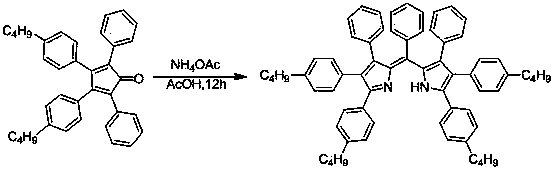
[0021] The preparation method of the pyrromethene fluorescent dye is as follows: put the cyclopentadienone derivative and the ammonium salt with a molar ratio of 1:4-200 into a sealed tube reactor or an autoclave, add an organic solvent and heat it in a sealed manner, and heat it at 100 React at -140°C for 2-24 hours at 1-10 atmospheres.
[0022] The structural formula of the cyclopentadienone derivative is , where Ar 1 is phenyl, C 1 -C 10 Alkyl substituted phenyl, C 1 -C 10 Halogen partially substituted and fully substituted alkyl phenyl, C 1 -C 10 Alkoxy substituted phenyl, fluorine, chlorine, bromine, iodine substituted phenyl, phenylethynyl or biphenyl; Ar 2 is phenyl, C 1 -C 10 Alkyl substituted phenyl, C 1 -C 10 Halogen partially substituted and fully substituted alkyl phenyl, C 1 -C 10 Alkoxy substituted phenyl, fluoro, chloro, bromo, iodo substituted phenyl, phenylethynyl or biphenyl.
[0023] The ammonium salt is one or more of ammonium acetate, ammoni...
Embodiment 1
[0026] Synthesis of (Z)-2,3,4-triphenyl-5-(phenyl(3,4,5-triphenyl-2H-pyrrole-2-ylidene)methyl)-1H-pyrrole
[0027]
[0028]The following steps are adopted: (1) Add 0.384 grams of tetraphenylcyclopentadienone derivatives in the above picture to a 15 ml sealed tube reactor, add 0.31 grams of ammonium acetate, add 2 ml of acetic acid, heat to 100 ° C, and react for 12 hours . Then cooled to room temperature, the reaction mother liquor was poured into 100ml of water, extracted three times with dichloromethane, the organic phases were combined, dried over anhydrous magnesium sulfate for 2 hours, and the solvent was removed by rotary evaporation. and then separated by column chromatography. Obtained dark red powdery solid 0.28 g, 82%. 1 H NMR (400 MHz, CDCl 3 ) δ 8.00 (s, 2H), 7.98 (s, 2H), 7.56 (s, 4H), 7.54 (s, 4H), 7.45 (t, J = 7.3 Hz, 4H), 7.41 (d, J = 7.1 Hz, 4H), 7.37 – 7.29 (m, 15H). MS (MALDI) ( m / z ): 676.2875 (M+).
Embodiment 2
[0030] (Z)-3-(3-methoxyphenyl)-2-((3-methoxyphenyl)(3-(3-methoxyphenyl)-4,5-diphenyl-2H -pyrrole-2-ylidene)methyl)-4,5-diphenyl-1H-pyrrole
[0031]
[0032] The following steps are adopted: (1) Add 0.444 grams of tetraphenylcyclopentadienone derivatives in the above picture to a 15 ml sealed tube reactor, add 1.54 grams of ammonium acetate, add 2 ml of toluene, heat to 110 ° C, and react for 24 hours . Then cooled to room temperature, the reaction mother liquor was poured into 100ml of water, extracted three times with dichloromethane, the organic phases were combined, dried over anhydrous magnesium sulfate for 2 hours, and the solvent was removed by rotary evaporation. and then separated by column chromatography. Obtained dark red powdery solid 0.29 g, 76%. 1 H NMR (400 MHz, CDCl 3 ) δ 14.95 (s, 1H), 7.49 (d, J = 6.5 Hz, 5H), 7.29 (m, 5H), 7.13 (m, 6H), 6.99 (m, 4H), 6.68 (d, J = 8.1 Hz, 2H), 6.44 (d, J = 8.4 Hz, 4H), 6.22 (d, J = 8.3 Hz, 4H), 5.92 (d, J = 7....
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com