Quinoline derivative, synthesis method of derivative and catalyst for synthesis
A synthesis method and technology of derivatives, applied in organic compound/hydride/coordination complex catalysts, physical/chemical process catalysts, chemical instruments and methods, etc., can solve the complex product purification process, low utilization rate of raw materials, treatment Cumbersome and other problems, to reduce the production of side reactions and impurities, shorten the reaction time, and achieve the effect of good biodegradability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
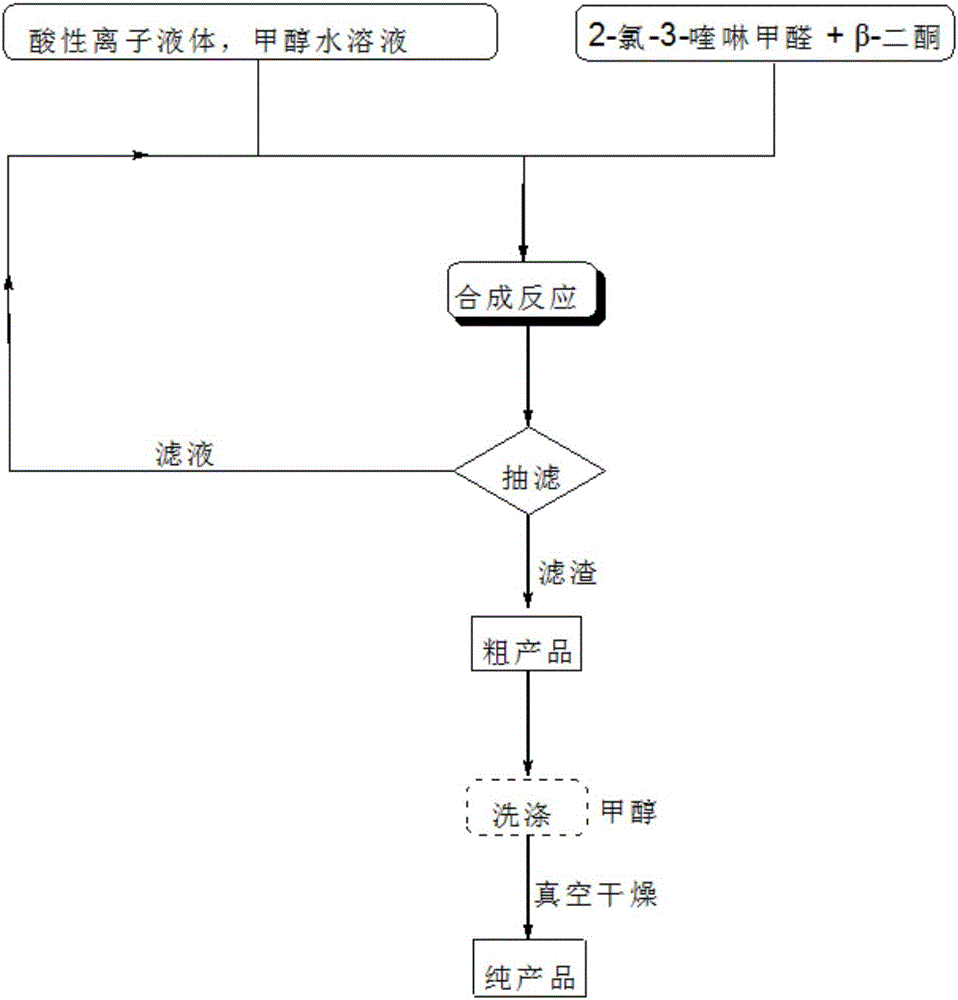
[0045] The flow chart of the synthetic method of quinoline derivative of the present invention is as figure 1 As shown, the specific steps are:
[0046] (1) Weigh the reaction raw materials 2-chloro-3-quinolinecarbaldehyde and β-diketone according to the molar ratio of 1:1.
[0047] (2) The weighed 2-chloro-3-quinoline formaldehyde and β-diketone are respectively added to methanol aqueous solution, and after fully dissolving and mixing, continue to add the molar amount of 2-chloro-3-quinoline formaldehyde 6~12% acidic ionic liquid catalyst, carry out heating reflux reaction under magnetic stirring condition, obtain solid precipitate, the volumetric amount of above-mentioned methanol aqueous solution in milliliter is 2-chloro-3-quinoline formaldehyde in millimole The molar weight is 7-10 times, the volume ratio concentration of methanol contained in the methanol aqueous solution is 88-93%, and the reaction pressure of the reflux reaction is 1 atmospheric pressure, and the refl...
Embodiment 1
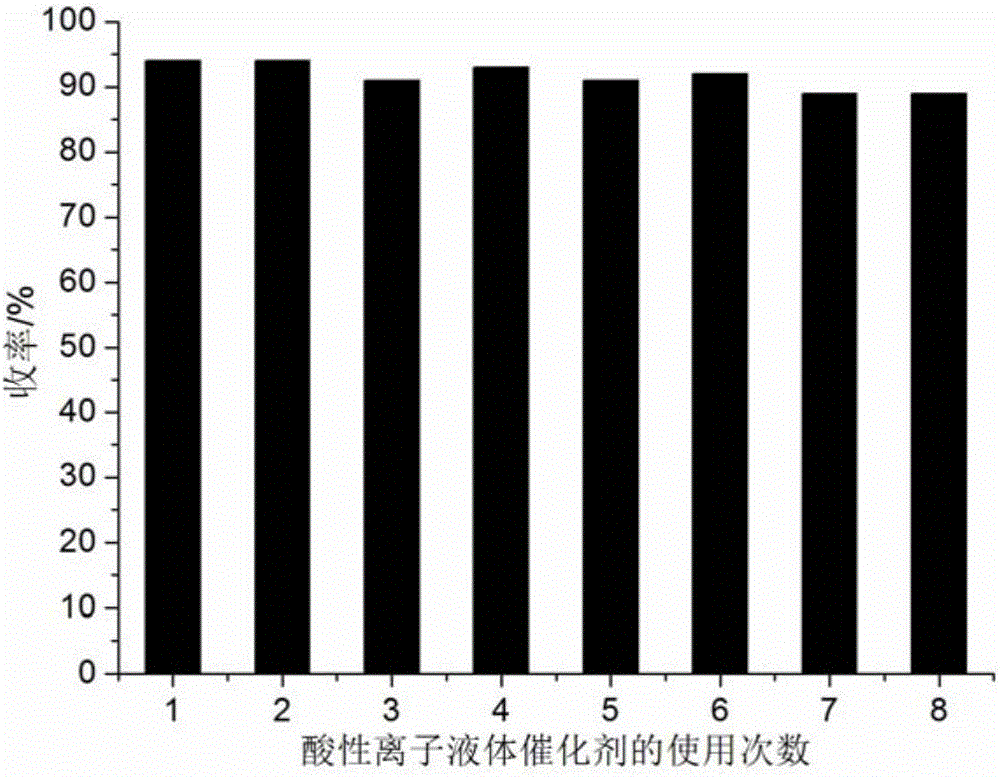
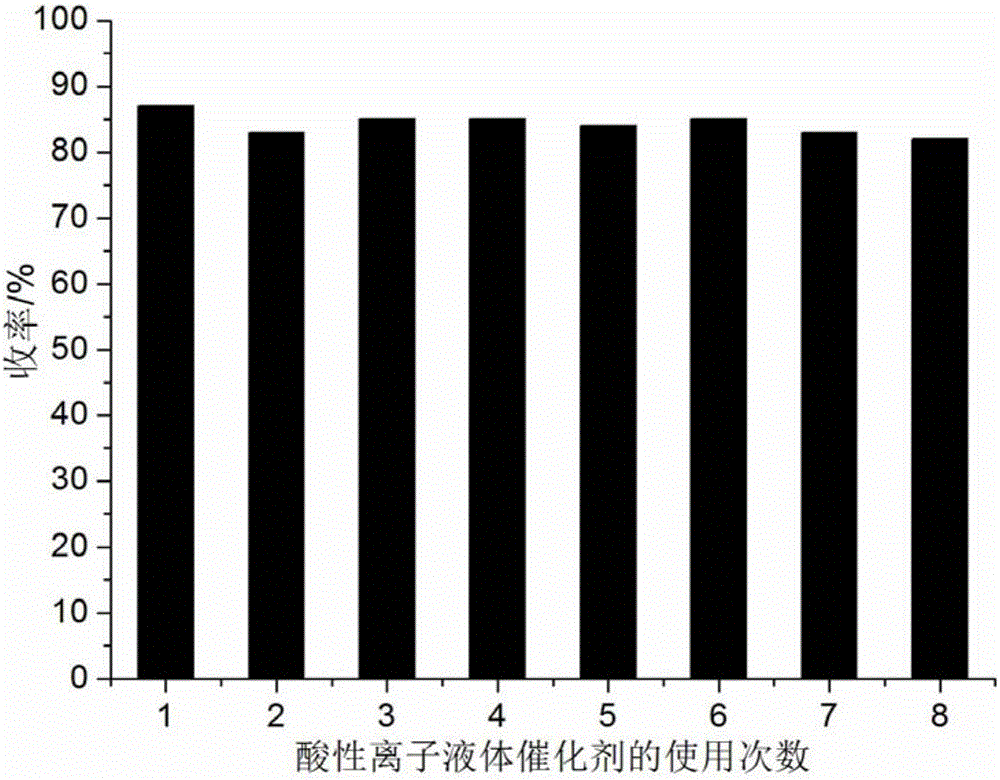
[0054] 1mmol of 2-chloro-3-quinolinecarbaldehyde, 1mmol of barbituric acid and 0.08mmol of acidic ionic liquid were respectively added to 9ml of 90% aqueous methanol in a 50ml single-necked flask with a stirring bar and a condenser. Heated to reflux for 12 minutes, TLC (thin plate chromatography) detection, the raw material point disappeared, cooled to room temperature, crushed the precipitated solid, left to stand for 2 hours, suction filtered, and the filter residue was washed with methanol and dried in vacuo to obtain 5-(2-chloroquinoline -3-yl)methylene-2,4,6-pyrimidinetrione, the yield is 94%, and the filtrate is directly added with 2-chloro-3-quinoline formaldehyde and barbituric acid for repeated use. The performance parameters of 5-(2-chloroquinolin-3-yl)methylene-2,4,6-pyrimidinetrione obtained in this example are as follows: m.p.>300°C; IR (KBr): 3447, 1721, 1539cm -1 ; 1 H NMR (400MHz, DMSO-d 6 ): δ=7.58(s, 1H), 7.93~8.02(m, 3H), 8.14(s, 1H), 8.30(s, 1H).
Embodiment 2
[0056] 1mmol 2-chloro-3-quinoline formaldehyde, 1mmol 2-thiobarbituric acid and 0.09mmol acidic ionic liquid were added respectively to the 50ml single-necked bottle containing 9ml 90% methanol aqueous solution with stirring bar and condenser . Heated to reflux for 14 minutes, TLC (thin plate chromatography) detection, the raw material point disappeared, cooled to room temperature, crushed the precipitated solid, left to stand for 3 hours, suction filtered, and the filter residue was washed with methanol and dried in vacuo to obtain 5-(2-chloroquinoline -3-base) methylene-2-mercapto-4,6-pyrimidinedione, the yield is 92%, after directly adding 2-chloro-3-quinoline formaldehyde and 2-thiobarbituric acid in the filtrate for reuse.
[0057] The performance parameters of 5-(2-chloroquinolin-3-yl)methylene-2-mercapto-4,6-pyrimidinedione obtained in this example are as follows: m.p.>300°C; IR(KBr):3441,1719, 1544cm -1 ; 1 H NMR (400MHz, DMSO-d 6 ): δ=7.26-7.32 (m, 1H), 7.36-7.41...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com