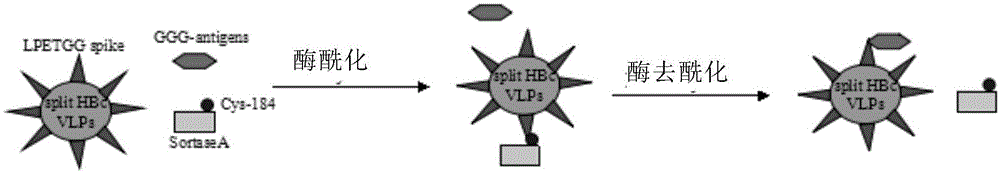
Method for preparing vaccine through transpeptidase shearing and application of vaccine
A technology of transpeptidase and antigen peptide, which is applied in the field of vaccine production technology, can solve the problems of reducing antigen activity, low chemical reaction specificity, and inconsistent antigen conformation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0100] Preparation of chimeric antigens
[0101] Currently, exogenous epitopes can be displayed on VLPs through gene fusion and chemical cross-linking. Gene fusion takes a long time, and the production and purification processes of different vaccines are complicated and costly. The method of chemical cross-linking combines non-covalent and covalent binding of exogenous antigen epitopes to the surface of VLPs, which is difficult to control the spatial structure of the antigen and may affect the antigenicity. In addition, the conditions of the chemical method are relatively harsh and the cost is high.
[0102] In the present invention, the hepatitis B virus core antigen (HEPATITIS B CORE ANTIGEN, HBcAg) is used as the vaccine carrier, and the HBcAg is cut off at the position of the antigenic determinant, divided into two peptide segments of N-terminal and C-terminal and expressed in Escherichia coli at the same time, both can Correctly folded virus-like particles (Virus-Like p...
Embodiment 1
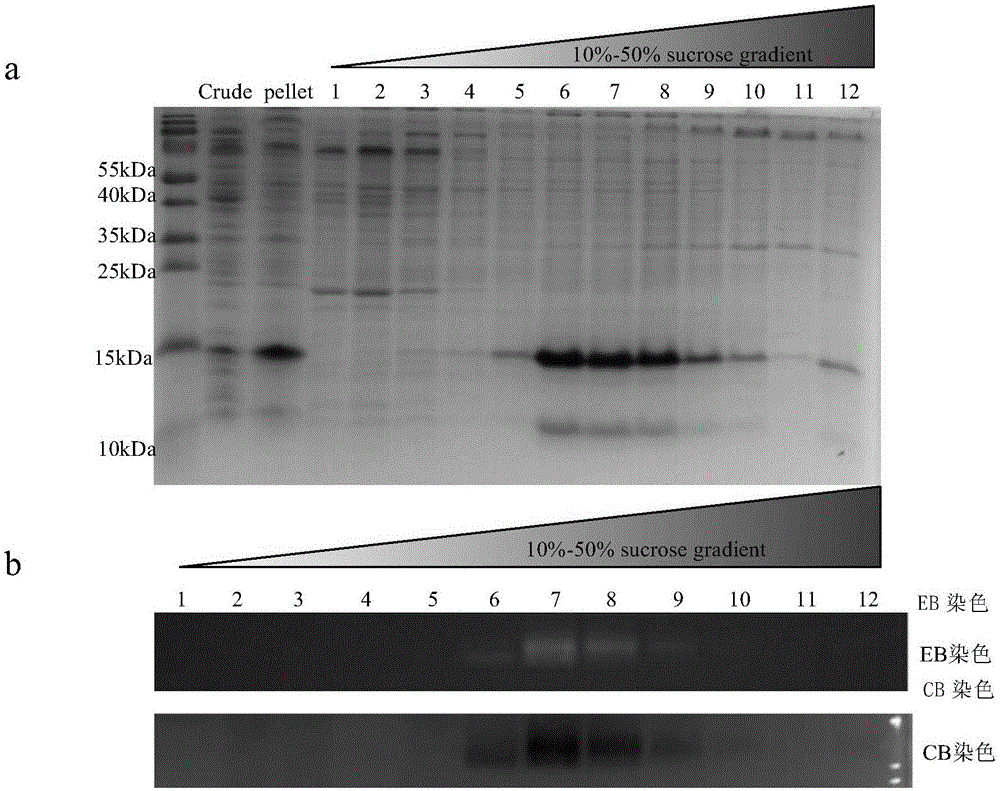
[0167] Example 1 Two-step purification of VLPs by ammonium sulfate precipitation enrichment and sucrose gradient separation
[0168] The genes of split HBc N-LPETGG-C and HBc-SP55 and HBc-SP70 used in the present invention were inserted into pET28a vector (purchased from Novagen), and then transformed into BL21plysS for expression. When the OD is 0.6-0.8, add 0.5mM IPTG and induce overnight at 18°C and 250rpm. The bacteria solution was centrifuged at 5000 rpm in a Beckman JLA10,500 rotor at 4°C for 10 min, and then washed once with 50 mM Tris pH7.5 and 150 mM NaCl (NT buffer). Transfer the bacterial solution to a 50ml centrifuge tube and centrifuge at 5000rpm, 4°C, 10min. Discard the supernatant, and the bacterial solution can be stored at -80°C for a long time. 1L of bacterial solution was resuspended with 30ml of NT buffer (50μg / ml of RNaseA was added), and ultrasonically disrupted: work for 5s, stop for 5s, a total of 30min. After the sonicated product was centrifuged ...
Embodiment 2
[0177] Example 2 Ni 2+ Column affinity purification and anion exchange two-step purification of transpeptidase and protein
[0178] The two proteins in the present invention: transpeptidase S-6xHis and GGGS-AD-4-6xHis are both expressed on the pET28a vector, transformed into BL21plysS. The induction conditions were the same as those for VLPs. The same goes for ultrasonic conditions. The centrifuged supernatant after sonication was subjected to Ni 2+ Column affinity purification and ion exchange purification. The buffers used are all NT buffers.
[0179] Ni 2+ Column affinity purification steps
[0180] 1. Add 30ml supernatant to NT buffer to balance Ni 2+ In the column, the shaker in the cold storage was mixed for 30min at a speed of 90rpm.
[0181] 2. Let the unbound Ni 2+ The solution from the column is gravity flowed, collected and labeled as flowthrough.
[0182] 3. Add 10ml of NT buffer containing 25mM imidazole for the first wash, marked as wash 1.
[0183] 4....
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com