Quality evaluation method for ganoderma lucidum medicinal material
A quality evaluation and technology of red ganoderma lucidum, applied in the field of quality control of red ganoderma lucidum medicinal materials, can solve the problems of rare and expensive reference substances, unable to represent the content of ganoderma lucidum acid, difficult production and circulation, etc., to facilitate identification, ensure consistency, and guarantee The effect of accuracy
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0046] Example 1 Preparation of Ganoderma lucidum extract reference substance
[0047] 1.1. Experimental overview
[0048] The solvent used in the preparation of the Ganoderma lucidum extract is methanol, and the extraction method is heating and refluxing.
[0049] 1.2. Experimental method
[0050] Two kinds of medicinal materials that were identified as red Ganoderma lucidum were separately prepared into Ganoderma lucidum extract. The preparation method is as follows: weigh 2kg of red Ganoderma lucidum medicinal material and crush, add 8 times the amount of methanol 16L, heat reflux and extract twice, each time 1 Hours (liquid boiling starts timing), filter, collect the filtrate, concentrate to 5L at 65°C under reduced pressure, add 120g of silica gel as auxiliary material, and continue to concentrate to dryness to obtain 200g of Ganoderma lucidum extract, respectively numbered No. 1 Ganoderma lucidum extract, No. 2 red Ganoderma lucidum extract.
[0051] Weigh 200 g of No. 1 Ganoder...
Embodiment 2
[0052] Example 2 Calibration of Ganoderma lucidum extract reference substance
[0053] 2.1 Experimental overview
[0054] Using ganoderic acid A, ganoderic acid B, ganoderic acid C2 and ganoderic acid G as reference materials, the content of ganoderic acid A, ganoderic acid B, ganoderic acid C2 and ganoderic acid G in the reference substance of Ganoderma lucidum extract was calibrated by liquid chromatography . The calibration method is as follows:
[0055] 2.2 Reagents
[0056] Ganoderma acid A, Ganoderma acid B, Ganoderma acid C2, Ganoderma acid G were purchased from Shanghai Yuanye Biotechnology Co., Ltd.
[0057] 2.3 Sample preparation of Ganoderma lucidum extract reference substance and ganoderic acid reference substance
[0058] Weigh 0.2g of Ganoderma lucidum extract reference substance, dissolve it with methanol and dilute to a 2ml volumetric flask. The sample is obtained by passing the 0.22um microporous membrane.
[0059] Weigh 5 mg of ganoderic acid A, ganoderic acid B, gano...
Embodiment 3
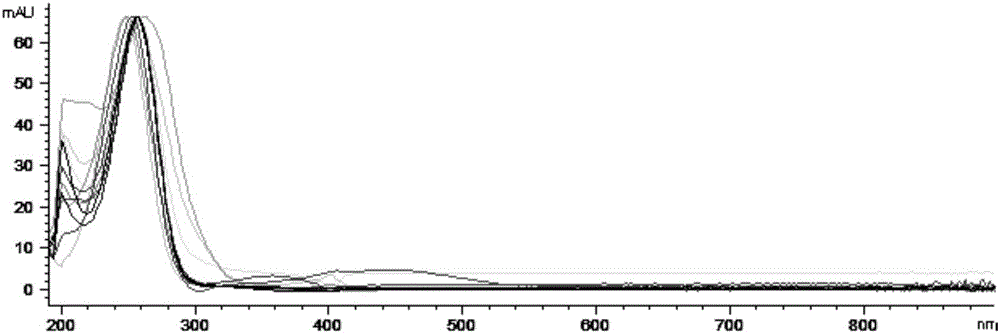
[0069] Example 3 The ultraviolet absorption analysis of the main components of the reference substance of the Ganoderma lucidum extract (retention time 15-65min)
[0070] 3.1 Experimental overview
[0071] The reference substance of Ganoderma lucidum extract was determined by liquid chromatography, and the ultraviolet absorption of the peak with the retention time of 15-65min in the liquid chromatography in the spectrum was analyzed.
[0072] 3.2 Sample preparation
[0073] Weigh 0.2g of Ganoderma lucidum extract reference substance, dissolve it with methanol and dilute to a 2ml volumetric flask. The sample is obtained by passing the 0.22um microporous membrane.
[0074] 3.3 Liquid chromatography conditions
[0075] Column: TSK gel ODS-80Tm (5μm 150*4.6mm)
[0076] Mobile phase: the following ratio, gradient elution
[0077]
[0078]
[0079] Flow rate: 1.2mL / min; Column temperature: 25℃; Detection wavelength: 254nm Injection volume: 10μl
[0080] 3.4 Experimental results:
[0081] Analyze ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com