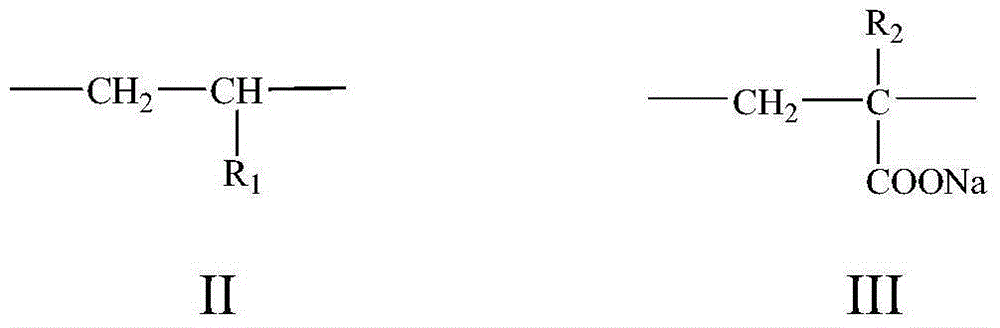Phenyl-containing hydrophobic cross-linking monomer and hydrogel based on phenyl-containing hydrophobic cross-linking monomer
A technology of cross-linking monomers and hydrogels, applied in the field of functional polymers, which can solve the problems of inability to solidify, failure of plugging, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
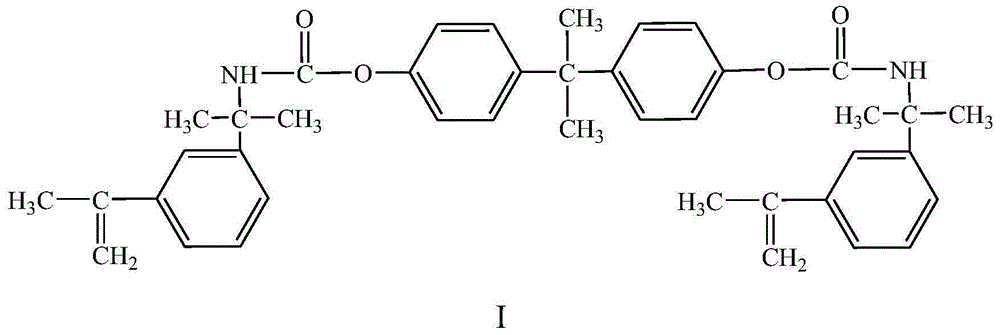
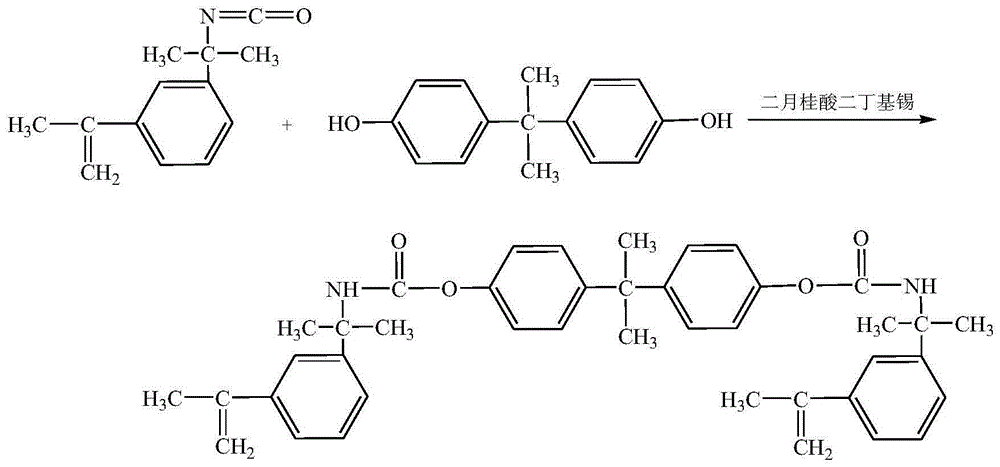
[0043] Preparation of Phenyl-Containing Hydrophobic Crosslinking Monomers
[0044]500 mL of acetone and 22.83 g of bisphenol A were added to a dry three-necked flask equipped with a stirrer, a condenser and a dropping funnel, and 42.26 g of 3-isopropenyl-dimethylbenzyl isocyanate was added to the dropping funnel. Stir until the bisphenol A in the flask is fully dissolved, then heat to 65° C. in a water bath, and apply vacuum for 2 hours while heating to remove moisture. Under nitrogen protection, 0.0338 g of dibutyltin dilaurate was added. The 3-isopropenyl-dimethylbenzyl isocyanate in the dropping funnel was dropped into the three-necked flask, the reaction was continued for 6 hours under stirring conditions, and the acetone was removed by distillation under reduced pressure to obtain the crude product of the target molecule. The crude product was rinsed three times with chloroform to remove unreacted monomers, and then placed in a 78° C. oven to constant weight to obtain th...
Embodiment 2
[0046] Preparation of hydrogels
[0047] 690g of 1,4-dioxane, 11.31g of N-isopropylacrylamide, 18.81g of sodium acrylate and 6.31g of the monomer prepared in Example 1 were sequentially added to the reactor, stirred until the monomer was completely dissolved, and the Nitrogen was introduced for 40 minutes to evacuate the air. 0.73 g of dibenzoyl peroxide was added, the temperature was raised to a predetermined reaction temperature of 86° C., and the reaction was stirred in a nitrogen atmosphere for 12 hours to obtain a pale yellow colloidal crude product.
[0048] The crude product was added to 1000 mL of ethanol for precipitation and then filtered to obtain a solid-phase product. Wash with acetone 3 times, and then use the glacial acetic acid-ethylene glycol mixed solvent with a volume ratio of 3:2 as the extraction agent to extract the product with a Soxhlet extractor for 24 hours, and vacuum dry at 25 ° C to constant weight, that is, obtain the target product.
Embodiment 3
[0050] Preparation of hydrogels
[0051] 812g of N,N-dimethylformamide, 12.72g of N,N-diethylacrylamide, 64.84g of sodium methacrylate and 12.61g of the monomer prepared in Example 1 were successively added to the reactor, and stirred until a single The body was completely dissolved and argon was bubbled for 60 minutes to evacuate the air. 3.60 g of dodecanoyl peroxide was added, the temperature was raised to a predetermined reaction temperature of 70° C., and the reaction was stirred in an argon atmosphere for 8 hours to obtain a pale yellow colloidal crude product.
[0052] The crude product was added to 1000 mL of ethanol for precipitation and then filtered to obtain a solid-phase product. Wash with acetone 3 times, and then use the glacial acetic acid-ethylene glycol mixed solvent with a volume ratio of 3:2 as the extraction agent to extract the product with a Soxhlet extractor for 24 hours, and vacuum dry at 25 ° C to constant weight, that is, obtain the target product....
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com